Social
Responsible Marketing
Responsible Marketing
Responsible Marketing Policy
Marketing Compliance
SK bioscience conducts responsible marketing in compliance with relevant laws and regulations, including the Pharmaceutical Affairs Act, the Medical Service Act, the Fair Trade Act, and the Code of fair competition. To this end, we have established compliance regulations and put in place detailed guidelines for providing economic benefits to healthcare professionals. We also contribute to protecting consumers and creating a fair competition environment in the industry by complying with laws and regulations related to fair trade and strictly regulating activities related to promotion and advertising. In particular, to prevent false and exaggerated advertisements, we specify the basic principles and scope of advertising for pharmaceuticals and established detailed compliance guidelines for quasi-drug advertising to minimize the possibility of product misuse. When producing promotional materials used by our marketing division, we use an internal review process called the RED system. It allows us to cross-check whether there are any violations from the medical, licensing, and legal perspectives. We do not conduct any marketing or promotion that may induce prescription of off-label products.
Monitoring Process for Responsible Marketing
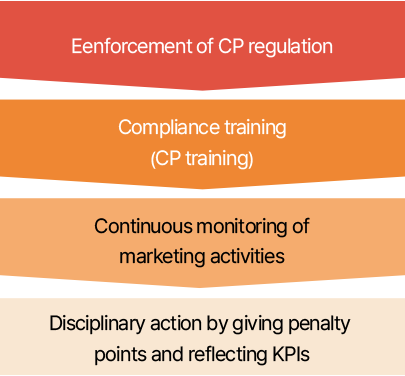
SK bioscience ensures that its sales partners participate in fair trade by conducting regular monitoring of marketing and promotional activities and obtaining fair trade agreements from them. To ensure compliance with marketing activities in advance, any expense discussions must be agreed upon by the Compliance Division. In addition, after expenses have been incurred, an expense report is prepared, and compliance with the fair competition code is checked through monitoring. If any violations are detected as a result of monitoring, penalty points are assigned, and disciplinary procedures are initiated in accordance with Article 14(Sanctions against Employees) of the Compliance Regulations. These results are then reflected in both organizational and individual KPIs and disclosed to all members.
Regular monitoring process for marketing and promotion activities

CP(Compliance Program) Check of Marketing Activities
-
Prior Arrangements
- Prior arrangements for CP obligations, approval of promotional activities
-
Report Submission
- Preparation and preservation of a report on expenditure details, including detailed evidence, in the system
-
Monitoring
- Monitoring of adequacy of marketing expenses
- Reflection of monitoring results in individual/organizational KPI evaluation
-
Feedback
- Feedback to violations of CP obligations
Marketing Compliance Training
SK bioscience conducts regular training sessions for relevant divisions on compliance with various marketing activities. We strive to establish a fair drug distribution order by providing detailed training on compliance regulations to be observed in marketing/digital marketing activities. The training covers the Pharmaceutical Affairs Act, the Fair Trade Act, and the Code of Fair Competition as well as how to write an expense report.
| Training Contents | Date |
|---|---|
|
January 18~19, 2022 |
|
July 21~22, 2022 Feedback · Feedback to violations of CP obligations |

