Business Ethics Policy
Code of Ethics and its Practice Guidelines
SK bioscience has established business ethics principles that reflect the basic management philosophy of the SK Management System (SKMS), with the belief that 'transparency is the key prerequisite for corporate sustainability'. The Code of Ethics, which was established in consideration of various stakeholders, serves as the basis for judgment of employees' decisions and behaviors in all management activities.
Code of Ethics
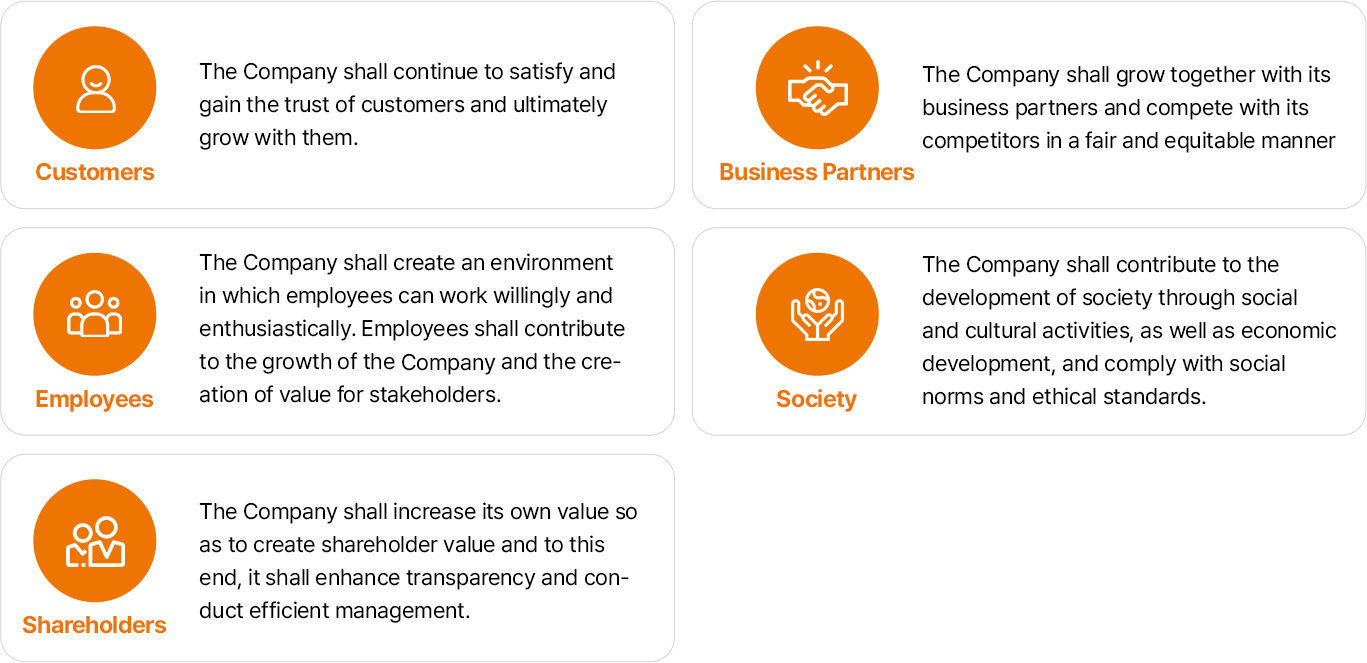
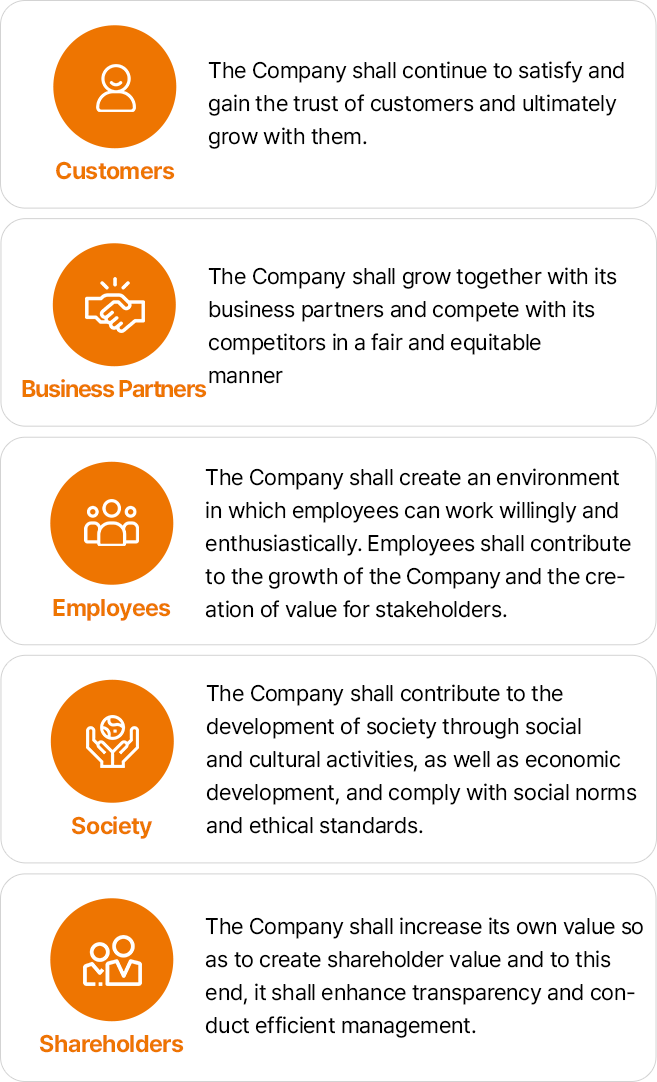
- 고객:회사는 고객을 지속적으로 만족시켜 고객으로부터 신뢰를 얻어야 하며, 궁극적으로 고객과 더불어 발전하여야 한다.
- 협력회사:회사는 협력회사와 공동발전을 추구하며, 정정당당하게 경쟁회사와 경쟁한다.
- 구성원:회사는 구성원이 자발적이고 의욕적으로 일할 수 있도록 환경을 조성하고 구성원은 기업의 발전 및 이해관계자의 가치창출에 기여하여야 한다.
- 사회:회사는 경제발전에의 기여와 함께 사회적 · 문화적 활동을 통하여 사회에 공헌하며, 사회규범과 윤리기준에 맞는 경영을 하여야 한다.
- 구성원:회사는 주주의 가치가 창출될 수 있도록 기업의 가치를 높여야 하며, 이를 위해 투명성을 제고하고, 효율적인 경영을 하여야 한다.
SK bioscience’s Code of Ethics Practice Guidelines outlines the ethical behavior and compliance obligations of our employees. All employees (including full-time, contract, and other non-exempt employees) adhere to these guidelines, and we actively encourage our stakeholders to do the same. Recognizing the need to supplement and revise the guidelines in recent years, we made efforts to refine them in 2022. The revised guidelines have become more specific and clear, and they now include case-based FAQs to assist employees in better understanding and utilizing them.
SK bioscience Code of Ethics Practice Guidelines
Attitude of Members
Sincere Execution of Work / Prohibition of Conflicts of Interest / Protection of Corporate Assets and Information / Prohibition of Offering or Receiving Money, Valuables and Entertainment / Mutual Respect among Members
Attitude toward Customers
Customer-centered Business Management / Protection of Customer Information
Responsibility for Members
Human-centered Management / Safe and Happiness of Members
Relationship with Business Partners
Win-win Management / Fair Competition with Business Partners
Responsibility for Society
Eco-Friendly management / Corporate Philanthropy
Compliance with Laws and Regulations
Compliance with fair trade laws and regulations / Compliance with international anti-corruption conventions and national · international laws and regulations
Responsibility for Shareholders
Enhancement of Corporate Value / Protection of Shareholder Rights and Interests
Application of Code of Ethics and Practice Guidelines
Compliance Obligations / Protection of Whistleblower
IDT Business Ethics Policy
IDT Biologika (hereinafter IDT) is committed to conducting its business with the highest ethical standards and in full compliance with relevant national laws, as well as the ten fundamental principles outlined by the UN Global Compact. This commitment applies to all employees across operational sites and to all business partners.
The IDT Code of Conduct outlines the fundamental principles for legally compliant behavior. It includes a strong commitment to respecting human rights and sets forth specific measures for protecting the environment and addressing climate change.
Business Ethics∙Compliance Management System
SK bioscience operates a business Ethics and compliance management system based on the belief that a company’s competitiveness can only be proven when it secures a competitive advantage in the market while adhering to laws and regulations. In addition, we practice business ethics and compliance management by implementing our Prevent-Detect-Respond framework. This approach helps us avoid profits from unethical business practices and lays the foundation for a fairer and more transparent company.
We set business ethics regulations and regularly conduct annual business ethics and compliance training and pledges to prevent risks and embed ethical practices in our employees. Additionally, we operate a monitoring system that continuously checks marketing cost execution. We conduct business ethics practice surveys to evaluate the level of ethical practices, and the results are reflected to improve the business ethics and compliance management system.
We also operate a reporting channel where employees, business partners, customers, and all other stakeholders can freely consult and report unethical behavior of the Company and employees, and if violations are confirmed as the result of investigation and monitoring of reports, we follow disciplinary procedures, notify all employees, and reflect them in regulations and trainings.
we established audit compliance regulations and secured specialized audit personnel to ensure independent audits in 2022. Building on this foundation, we conducted regular audits focusing on cost and procurement areas in 2023, and further checked key expense items for potential misuse, reporting the results to the Audit Committee in 2024. We will continue to audit key areas of vulnerabilities to identify and remediate management leaks and inefficiencies.
Business Ethics∙Compliance Management Procedure
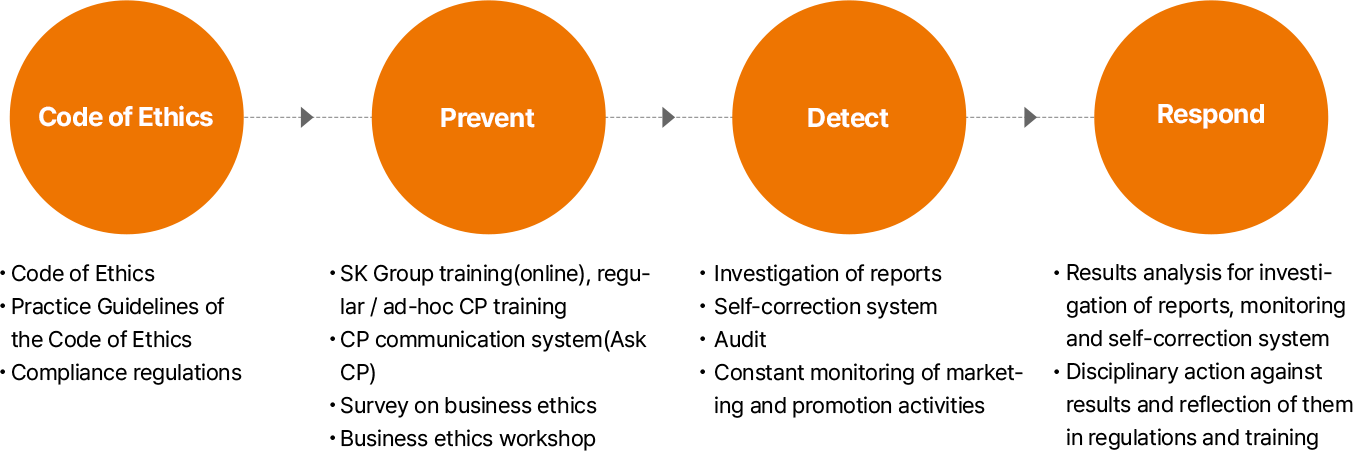
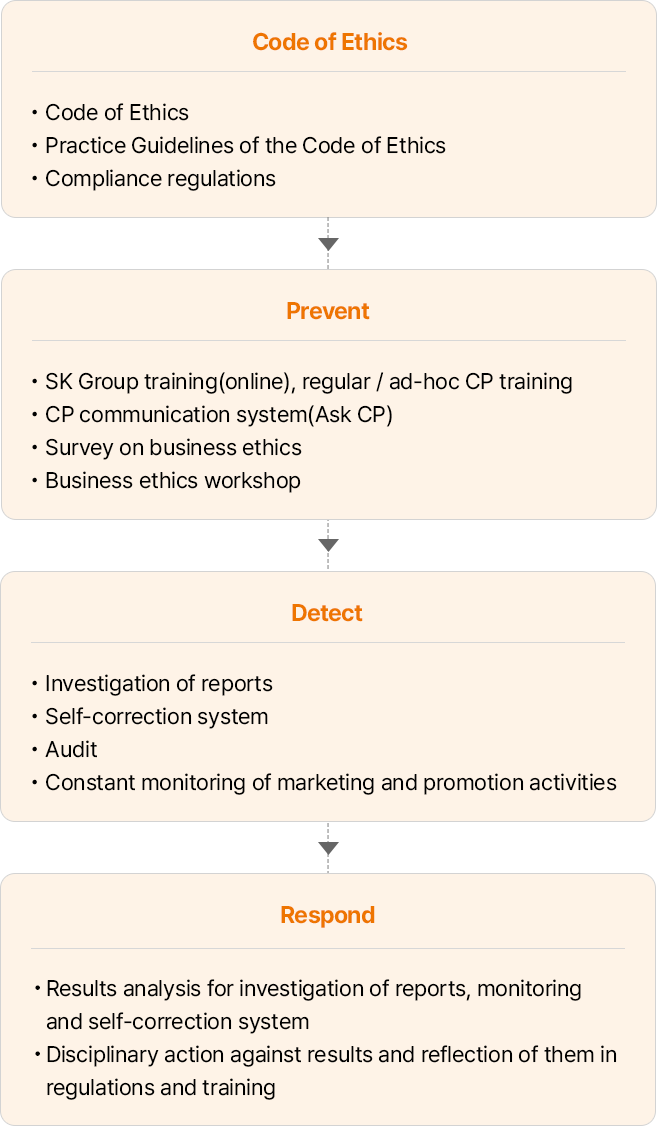
- 윤리규범 • 윤리규범 • 윤리규범 실천지침 • 컴플라이언스 규정
- Prevent • 그룹 온라인 교육, 정기/수시 CP교육 • CP comm. 시스템 (CP 물어보세요) 운영 • 윤리경영 실천 설문조사 • 윤리경영 실천 워크숍
- Detect • 제보조사 • 자정시스템 • 감사 • 마케팅 판촉활동 상시 모니터링
- Respond • 제보조사, 감사, 자정시스템, 모니터링 결과 분석 • 결과에 대한 징계 및 규정 · 교육 반영
Business Ethics∙Compliance Implementation Governance
SK bioscience has established a company-wide business and compliance management system and operates related programs. The Head of Legal & Patent Office is appointed as the Compliance Officer, responsible for planning, implementation, evaluation, and supervision of the entire process of the voluntary compliance program. In addition, the Business Ethics and Compliance Officer oversees business-related activities such as pharmaceutical compliance activities and programs to raise the level of awareness of voluntary fair trade compliance among all employees. The Compliance Officer reports annually to the Board on these activities and future plans.
We operate a dedicated compliance division (Ethics Management Team) to establish a compliance system, monitor and investigate reports. The Team also makes efforts to prevent compliance violations in advance by providing compliance-related training and imposing penalties for violations. In addition, in order to review the internal business system as the Company's business environment changes, we secured specialized audit personnel and added audit functions to the Ethics Management Team in 2022 to establish audit system. Since 2023, we have made every effort to upgrade the business ethics and compliance management system throughout the Company by conducting full-scale audit activities.
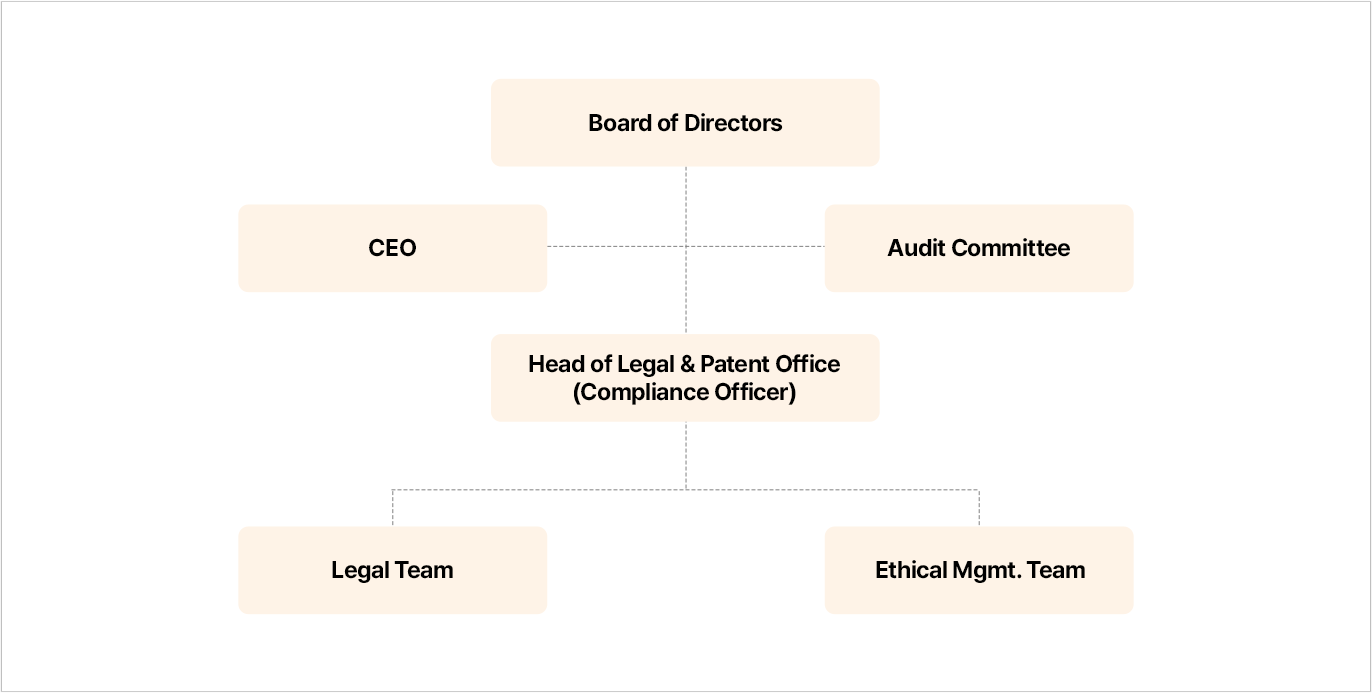
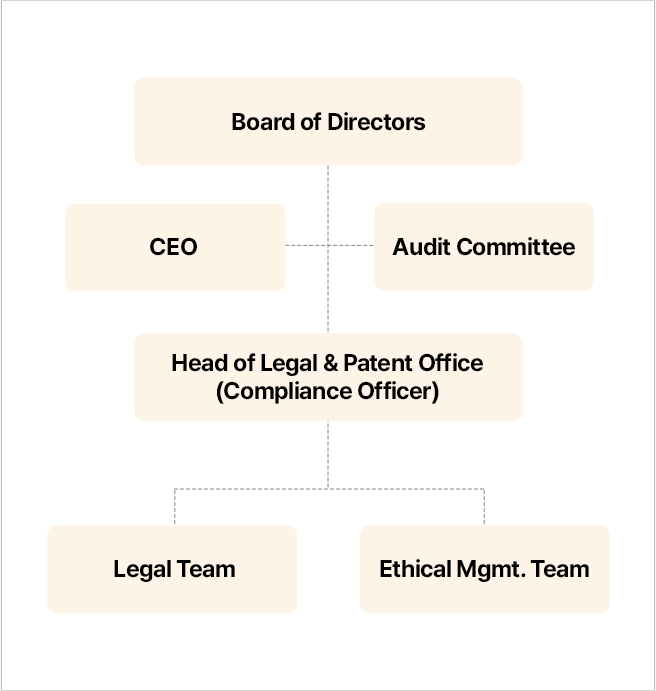
- Board of Directors
- 대표이사, 감사위원회
- 법무특허실장(준법지원인/자율준수관리자)
- 법무팀, 윤리경영팀
Responsibilities of Compliance Officer
SK bioscience has appointed the head of the Legal & Patent Office as the Compliance Officer to practically carry out compliance control activities. The tenure for the Compliance Officer is three years, and he/she plays a key role in overseeing the Company's compliance works, strengthening the governance system and proactively preventing legal risks.
SK bioscience has strengthened its compliance system by specifying the activities, duties, and authority of the Compliance Officer in the Compliance Control Standards. The Compliance Officer is responsible for operating compliance education/training programs and checking whether the Guidelines are being followed. If necessary, the Compliance Officer may request employees to submit materials and seek advice from external experts, as well as make a statement to the Board in relation to compliance control. In addition to general compliance activities, the Compliance Officer strives to advance the company-wide compliance system through risk prevention activities, thereby contributing to the achievement of the Legal and Patent Office's 2030 long-term vision of becoming a “Proactive & Business-oriented Legal Solution Provider.
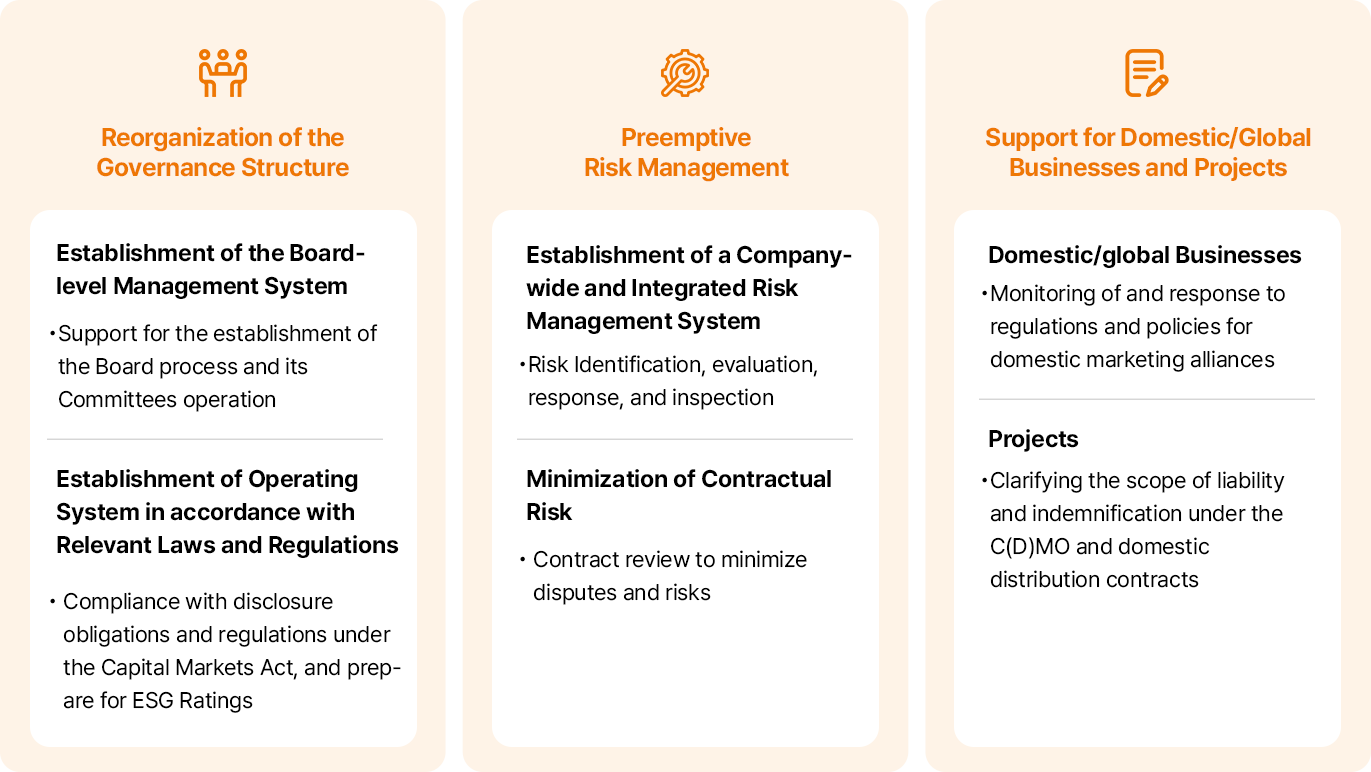

-
거버넌스 체계 정비
- 이사회 중심 경영 정착
- 이사회 프로세스 정착 및 산하 위원회 운영 지원
- 관련 규제 〮 법령에 부합하는 운영체계 수립
- 공시 의무, 자본시장법상 규제 준수, ESG 평가 대비
-
선제적 리스크 관리
- 전사적/통합적 리스크 관리 체계 구축
- 리스크 식별, 평가, 대응, 점검
- 계약상 리스크 최소화
- 분쟁 및 당사 리스크를 최소화하기 위한 계약 검토
-
국내외 사업 및 프로젝트 지원
- 국내외 사업
- 국내 마케팅 협약(Alliance) 규제 및 정책 모니터링, 대응
- 프로젝트
- C(D)MO, 국내 유통 계약 시 당사의 책임 및 면책 범위 명확화
IDT Business Ethics ∙ Compliance Management System
A key component of IDT business ethics and compliance framework is the regular conduct of audits and risk assessments. These assessments focus on identifying and evaluating potential risks in areas such as:
- Economic Crime: Including anti-corruption, bribery, and money laundering
- Protection of Sensitive Data and Business Secrets
- Compliance with Competition and Antitrust Laws
The objective of these audits is to systematically identify potential risks, implement appropriate measures to address those risks, and ensure that the entire process is fully documented and traceable. Additionally, IDT conducts a risk-based analysis of its business partners, known as the Business Partner Review.
In line with the EU Whistleblower Directive 2019/1937 and with the approval of the works council, IDT introduced an electronic whistleblower system in 2021. This system allows both employees and external third parties to report violations of compliance regulations, human rights infringements, environmental law breaches, and other misconduct.
Business Ethics∙Compliance Capacity-building
SK bioscience administers various forms of ethics training for all employees (including part-time) and contractors, to ensure comprehensive understanding and adherence to the Code of Ethics. SK Group's online ethical management training utilizes video contents based on various cases such as sincere execution of work, mutual respect, asset protection, conflicts of interest, and acceptance of money, valuables and entertainment to strengthen employees' ethical and compliance capabilities and internalize them into ethical decision-making. SK Group also conducts ethical management practice workshops at each organization where leaders and employees discuss ethical dilemmas and ways to practice business ethics together. Furthermore, the Company implements regular compliance training for the sales and marketing divisions and delivers ad-hoc training during the onboarding of new and experienced staff to fortify a robust internal compliance culture.
In order to raise employees' awareness of ethical practices, we first implemented company-wide ethical management self-education in 2023, and in 2024, we conducted self-education based on key ethical management issues for all employees.
Ethics Training
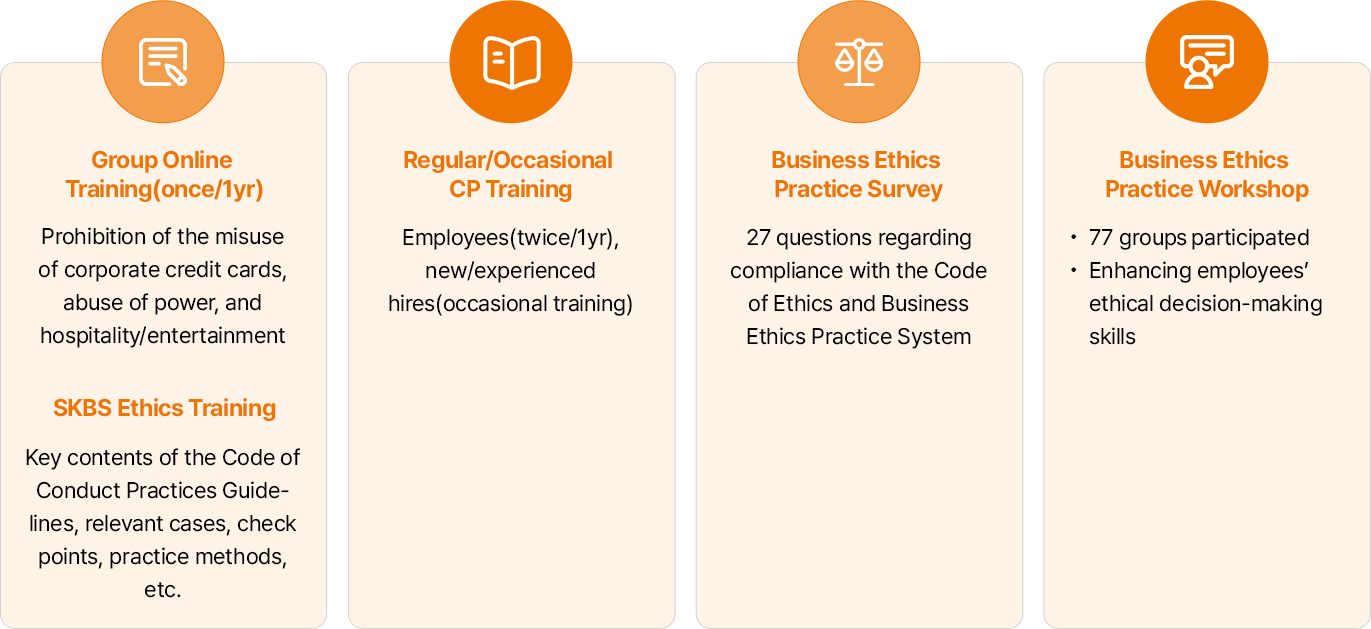

- 그룹 온라인 교육(연 1회):법인카드 편법 사용, 겸업금지, 갑질, 접대/향응 금지 등
- 자체 윤리경영 교육(연 1회):윤리규범실천지침 주요 내용, 발생 사례, Check Point, 윤리실천 방법 등
- 정기/수시 CP 교육:재직자(연 2회), 신규/경력 입사자(수시 교육)
- 윤리경영 실천 Survey:윤리규범 준수, 윤리경영 실천 제도 관련 27개 문항
- 윤리경영 실천 Workshop:- 77분과 토론식 교육 - 딜레마 상황에서의 구성원의 윤리적 의사결정 능력 제고 교육
IDT Business Ethics ∙ Compliance Capacity-building
To ensure the effective integration of ethics and compliance management system, IDT provides specialized training for two distinct employee groups. All new employees are required to complete this training as part of their onboarding process. Additionally, existing employees are required to undergo regular refresher courses. The specifics of the training methodology and the selection of topics are outlined in the "IDT Biologika GmbH Guidelines for Compliance Training.“
