Responsible Research & Development
Safe Clinical Research
Complying with International Code of Ethics
SK bioscience conducts safe clinical research in accordance with international ethical regulations. We follow the ICH1) guidelines and KGCP2) which is domestic guidelines for conducting clinical trials. Other clinical trials are conducted after obtaining approvals from drug regulatory authorities in accordance with the regulations of the country in which they are conducted. We also comply with the principles contained in the Declaration of Helsinki, which are ethical principles for medical research involving human subjects.
- 1)ICH : International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- 2)KGCP : Korean Good Clinical Practice
- 전임상
- 암성1상
- 암성2상
- 암성3상
- 허가
Securing Clinical Trial Safety and Transparency
SK bioscience ensures the safety of clinical trials by strictly complying with the regulations and guidelines of Korea’s Ministry of Food and Drug Safety and the respective countries where the trials are conducted. In the preclinical stage, toxicity is tested in advance through animal testing, and the safety of subjects and the efficacy of the vaccine are evaluated at each stage of the clinical trials.
We ensure the transparency of our clinical trials by collecting and submitting all data on clinical trial approvals to the relevant regulatory authorities. Specifically, we prepare reports on serious adverse events that occur during the clinical process and report them to each regulatory authority. In addition, information on all interventional studies conducted by SK bioscience is registered and disclosed on the clinical trial information site operated by the U.S. National Library of Medicine, in accordance with the progress of the clinical trial.
Pursuing Diversity in Clinical Trials
The diversity of participants in clinical trials is an important factor not only for product development and success but also for equity. Recognizing this, SK bioscience conducts global clinical trials in various countries, including Korea, to collect data of participants from different races/ethnicities, ages, genders, and backgrounds. In the case of SKYCovione, one of the vaccine pipelines developed by SK bioscience, we have received the approval based on data from Southeast Asia, Oceania, and Europe, in addition to Korea. Based on the safety data of SKYCovione, our aim is to secure diversity in terms of countries and ages in clinical trials for the next vaccines.
Clinical Trial Participant Safety & Practices
SK bioscience is committed to protecting the safety and rights of all clinical trial participants. Participants are asked to participate through a voluntary consent process after fully understanding the risks and benefits of clinical trials. Moreover, blood specimen collection is conducted with additional consent after providing sufficient information to subjects through a separate human-derived material informed consent form.
We have also established standards and procedures to compensate for damages that may occur during the clinical trial phase and have implemented safeguards for participants through the operation of the DSMB3). The DSMB consists of external and independent experts responsible for advising on whether to continue, modify, or terminate the clinical trial. They conduct periodic reviews and evaluations of the trial process, safety data, and, if necessary, critical efficacy endpoints. The Board plays a crucial role in protecting the safety and rights of participants, including the decision to terminate a trial early after assessing the risks and benefits for participants.
In the process of conducting clinical trials, we have taken measures to ensure that participants can progress to the next stage only after completing safety reviews at each trial stage. We also adhere to standards that are stricter than international standards, such as setting the follow-up monitoring period to one year.
- 3)DSMB : Data Safety Monitoring Boards
Clinical Trial Quality Enhancement
Clinical Audits
SK bioscience conducts clinical audits as a procedure to ensure that clinical trials are conducted in accordance with ethical principles and standards. We conduct clinical audits in accordance with internal regulations such as protocols and SOPs, as well as domestic and international guidelines such as GCP1) and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, regulations of the IRB2), and the Helsinki Declaration.
The Development QM Team ensures its objectivity and credibility by conducting independent audits. The Development QM Team sets a Quality Management Plan (QMP) and establishes a detailed approach for each clinical audit based on the plan to assess the appropriateness of the study’s operations. Under the plan, the Team conducts in-house audits of materials and related documents to review the clinical trial process, safety and validity of data, appropriateness of documentation, and compliance with regulations. Also, the Team conducts on-site audits of clinical trial sites analyzing and reviewing the gathered information and reports the results after the audits. By doing so, we aim to ensure that the principles of protecting the rights and welfare of clinical trial subjects, beneficence, and justice are upheld. Moreover, we hope to improve systems and procedures in the new drug development process, and ultimately advance data quality.
Additionally, SK bioscience actively fulfills its supervisory role as a market authorization holder and clinical trial sponsor by strengthening the qualification evaluation and regular audit procedures for partner companies involved in clinical trials. This ensures the professionalism, reliability, and performance capabilities of these partners, enhancing the overall quality and integrity of the clinical trials conducted.
- 1)GCP : Good Clinical Practice
- 2)IRB : Institutional Review Board
Strengthening Clinical Audits Capabilities
The Development QM team which is SK bioscience’s clinical audit division is composed of internationally certified auditors and specialized personnels dedicated to the clinical trial quality of global clinical trial. We are strengthening our clinical audit capabilities and the quality of clinical audits by providing key personnel with opportunities to participate in various seminars, conferences, and external trainings both domestically and internationally.
Additionally, SK bioscience aims to ensure its expertise as a global player and ultimately enhance its international competitiveness by continuously increasing the number of certified “Reliability Assurance Professionals”.
Improvement of the Clinical Trial Quality Management System
Recently, a risk-based approach has become prevalent in the pharmaceutical and bio-industries. Clinical trial regulations have been strengthened as the risks must be identified, evaluated, and reported during the clinical trial process. As the number of our research projects conducted abroad and business partners at home and abroad grows, so does the importance of clinical trial quality assurance and management.
Accordingly, SK bioscience operates a QMS3) including quality control and quality assurance activities for clinical trial data, training to strengthen employee competencies, and SOP management to improve the efficiency and accuracy of clinical trial quality management. SK bioscience digitized the QMS to reduce the time and cost spent and established a foundation to more actively cope with changes in the regulatory environment at home and abroad.
- 3)QMS : Quality Management System
Quality & Safety Management
Quality Management System
SK bioscience operates a strict quality management system that covers all stages of the product life cycle, from product development to consumer use. Our quality assurance system is optimized to continuously produce, manage, and sell pharmaceutical products in a GMP1) environment. In addition, we manage issues such as deviations and complaints with a system to produce high-quality pharmaceuticals.
We also perform qualification and validation of all facilities and equipment to ensure their performance and functionality. As such, we have established and operated a quality policy to comply with all customer and legal requirements, as well as standards for quality assurance, facilities/equipment, raw materials, manufacturing control, packaging/labeling, and test management. Moreover, we put our efforts for the growth of our employees, and we are constantly striving to establish a quality-first culture.
- 1)GMP : Good Manufacturing Practice
Quality System
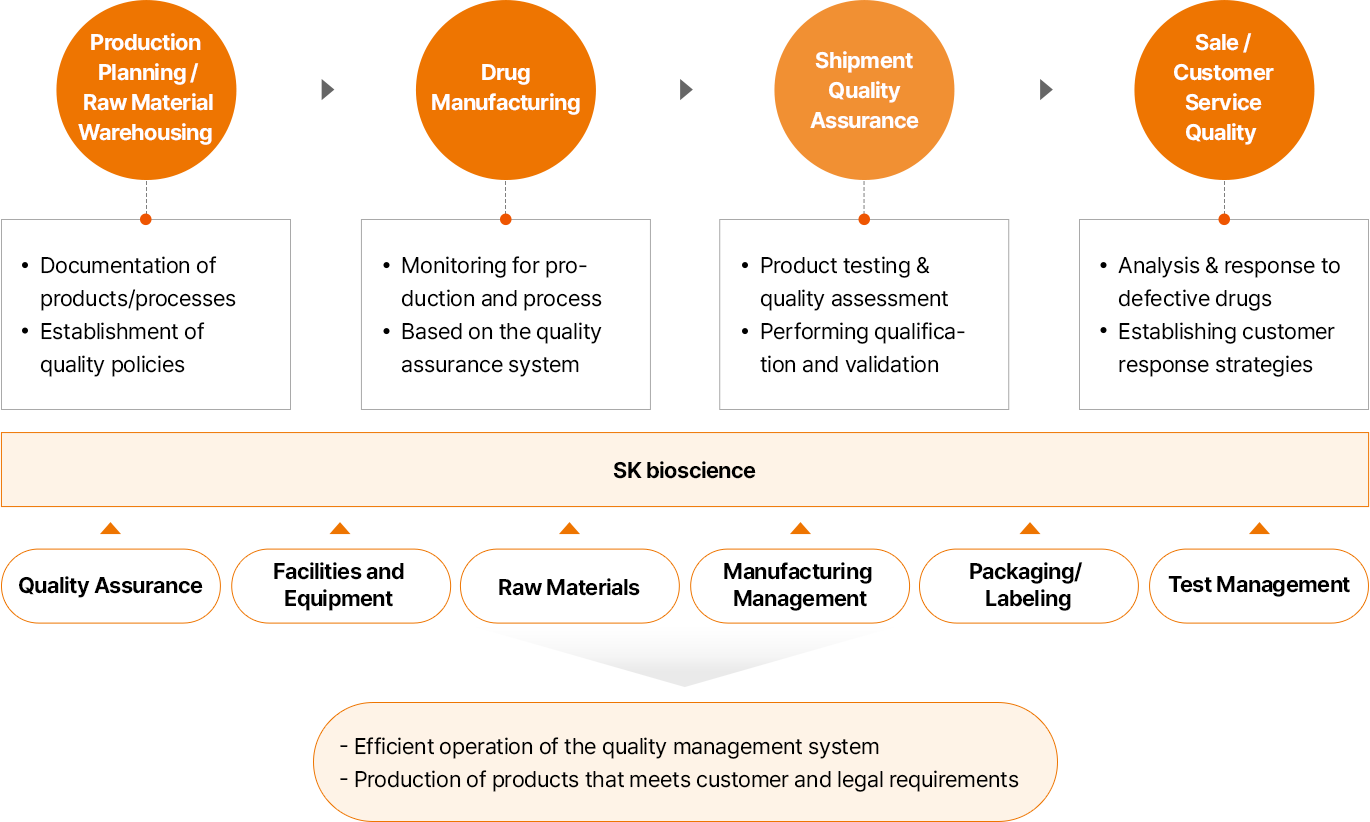
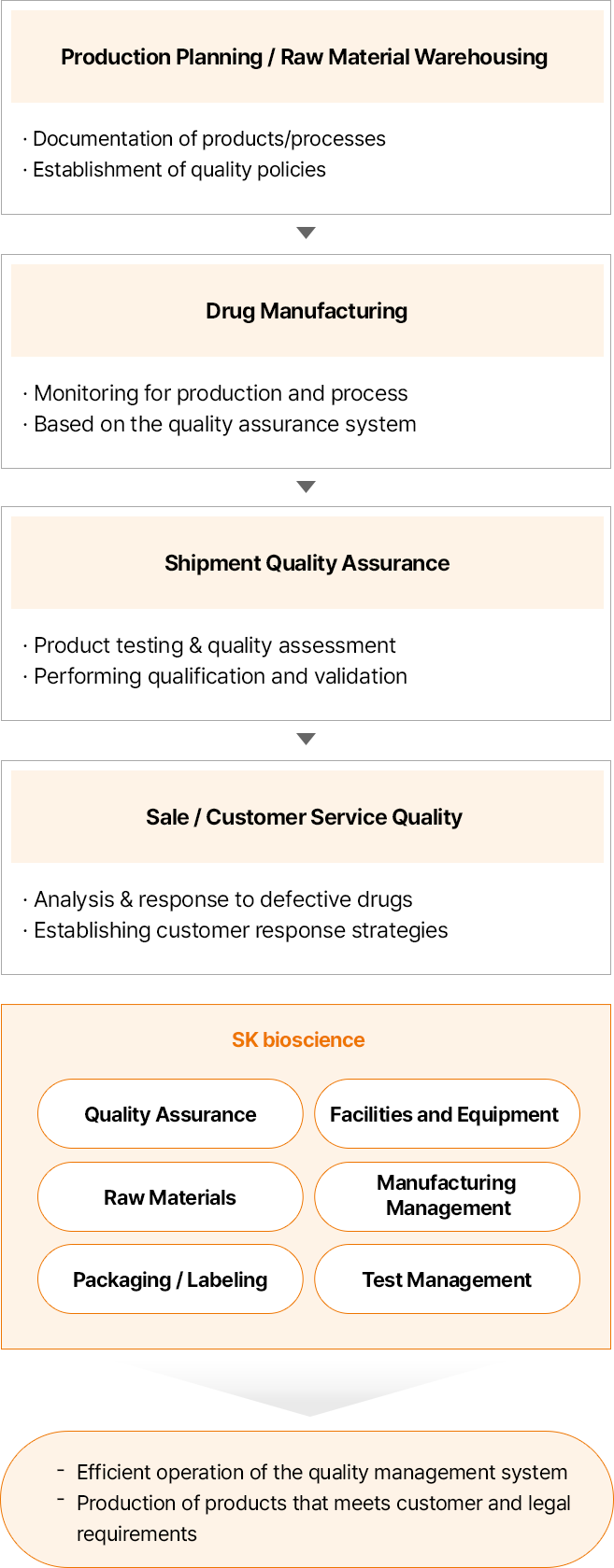
-
- 생산 계획 / 원자재 입고
- 제품/공정 문서화
- 품질 정책 수립
-
- 의약품 제조
- 생산 및 공정 모니터링 수행
- 품질보증 시스템 기반
-
- 출하 품질 보증
- 제품 시험 및 품질 평가
- 저격성 평가 및 밸리데이션 진행
-
- 판매 / 고객 품질
- 불량 분석/대응
- 고객 대응 전략 수립
- SK바이오사이언스
- 품질보증
- 설비 및 장비
- 원자재
- 제조관리
- 포장/라벨
- 시험관리
- 품질경영시스템의 효율적 운영
- 고객 요구사항과 법적 요구 사항을 충족하는 제품 생산
Quality Management Implementation System
SK bioscience has a system in place to ensure the smooth operation of the quality assurance system and GMP. Based on its annual education and factory quality training system, the Quality Assurance (QA) Division supports risk analysis for quality management, performs deviation handling procedures, and operates a preventive action system. It also conducts evaluation and monitoring for installation, calibration, and maintenance of GMP-compliant facilities and equipment. Moreover, it oversees general quality management tasks such as product recall notification/investigation/record storage, supplier audit, and purchase change approval.
The Quality Control (QC) Division examines raw materials, intermediate process samples, and final drug substances used in pharmaceutical manufacturing to check whether the specifications have been met. In this process, physics and chemistry, microbiology, animal testing, and device analysis tests are conducted according to the Standard Operating Procedure (SOP). To this end, it carries out test method validation and management activities to maintain the functions of the QC laboratory, such as device qualification and CSV2). We are committed to continuous improvement of our quality system by monitoring our drug safety evaluation programs and manufacturing support facilities, regularly reporting the results to top management, and receiving feedback from management.
- 2)CSV : Computer System Validation
SK bioscience Quality Policy
- 1The Quality Division and the Production Division must be operated independently.
- 2All employees who perform GMP must have appropriate qualifications, improve, and maintain GMP level through training.
- 3All important manufacturing processes must be validated, and the quality standards established through validation must be reviewed regularly and kept up to date.
- 4All the facilities and systems required for GMP must be equipped.
- 5All work during the manufacturing process must be performed according to the approved method. All progress must be recorded and preserved in the manufacturing instructions and records.
- 6A recall system must be established and maintained.
- 7A complaint handling system must be kept in place, and the cause of complaints that arise should be investigated. Appropriate measures to prevent the recurrence of the same complaints must be taken.
Quality Assurance Enhancement
SK bioscience operates a quality management process that oversees and guarantees the pharmaceutical manufacturing process from raw material intake to final product shipment. By establishing and maintaining a quality management system, the Company ensures that it provides reliable products to customers. SK bioscience complies with the Korean Good Manufacturing Practice (K-GMP) standards across all its production facilities. Additionally, for products approved in international markets, SK bioscience has obtained EU-GMP certification from the European Medicines Agency (EMA), certification from the UK's Medicines and Healthcare products Regulatory Agency (MHRA), and WHO Prequalification (WHO PQ) for its production sites. These certifications ensure that all production facilities meet internationally recognized third-party quality standards.
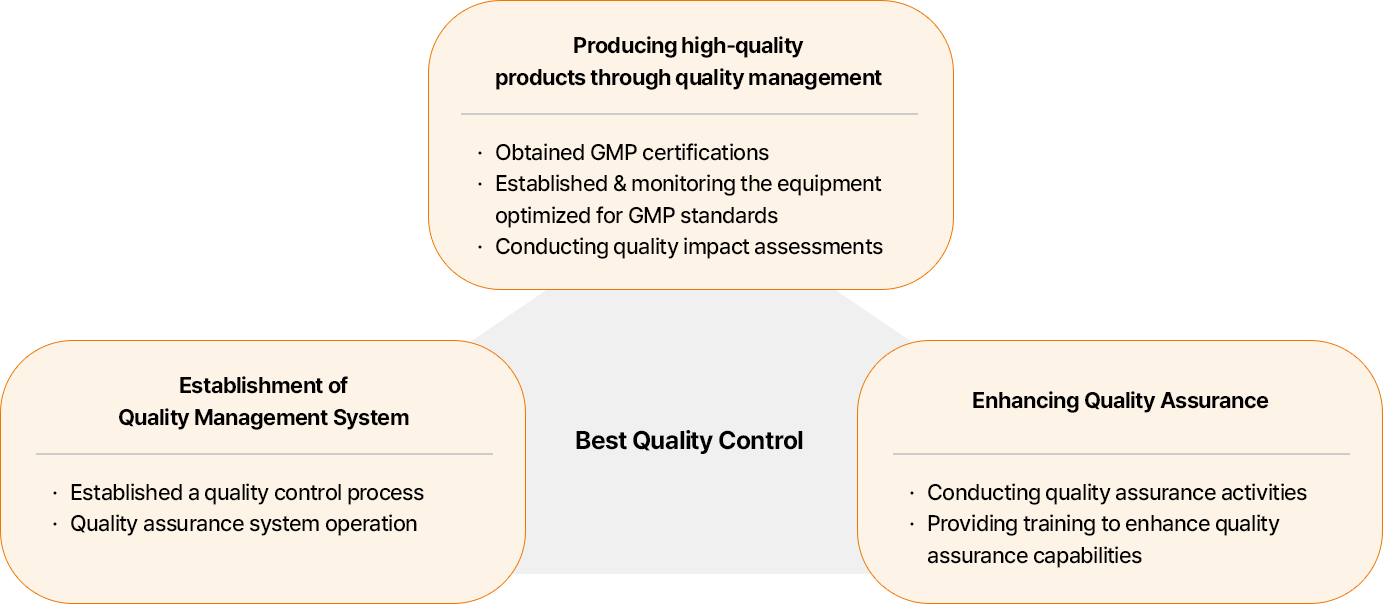
Best Quality Control
- 품질경영 실행을 통한 고품질 제품 생산:GMP 인증 획득, GMP 기준 최적화된 설비 구축 및 모니터링, 품질 영향평가 시행
- 품질경영시스템 구축: 품질관리 프로세스 수립, 품질보증 시스템 운영
- 품질보증 강화:품질보증 활동 수행, 품질보증 역량 강화 교육 진행
Quality Assurance Activities
SK bioscience pays special attention to diagnosing and managing product quality risks as we produce vaccine raw materials and finished drugs that are sensitive to temperature and environment. Products are stored and monitored at specified temperatures and environments. Commercialized products undergo annual safety tests to check their quality. Raw materials are also managed under proper storage conditions. In addition, for GMP compliance and process control, critical processes are managed and supervised on-site based on product standards, manufacturing records, and a guidebook. Moreover, QA on the shop floor policy1) is implemented to ensure data integrity, and process deviations are handled on-site in real-time.
We continuously manage whether quality issues are improved and whether operations are in line with global GMP standards by conducting a quality impact assessment of products in the first half of each year. A validated eQMS is applied to the quality assurance system, allowing for comprehensive management of changes, deviations, corrections, and prevention, as well as deviations from standards, to be tracked in real time.
- 1)The QA on the Shop Floor Policy : A system that enables quality assurance personnel to participate directly in key processes to solve or respond promptly to problems when they occur. It is operated to prevent unnecessary process deviations.
Enhancing Quality Assurance Capabilities
We conduct trainings for strengthening quality assurance capabilities of product quality managers. We conduct regular trainings to strengthen GMP competencies. And new employees receive basic training and on-the-job training to ensure they have the necessary skills for their respective jobs.
Jobs that require special capabilities, such as quality testing and aseptic work, are strictly managed to ensure that only authorized employees perform such tasks through verification and qualification procedures. Therefore, our training programs provide not only trainings on common competencies such as quality system operation, production procedures, hygiene, and dress code but also detailed trainings for each specific job, as well as training to enhance the capabilities of each employee.

GMP Training Poster

Training for Strengthening Quality Assurance Capabilities
Quality Test Management
SK bioscience ensures the reliability and management of quality tests for raw materials, intermediates, and final products using validated test methods, trained personnel, and certified equipment. To guarantee the quality and safety of all manufactured pharmaceuticals, we conducts precautionary tests for emerging quality and safety concerns. Also quality tests are conducted before shipment, with results reviewed and managed by the Quality Assurance Division.
The tests are categorized into physicochemical, viral, microbiological, and animal tests. Certified personnel conduct these tests using pharmacopeia standards (USP, EP, KP, etc.)2) and validated test methods. A dedicated stability chamber3) is in place for stability studies, with continuous monitoring to collect stability data.
- 2)Pharmacopeia: Comprehensive collections of detailed information for pharmaceutical quality management, published by various countries, with prominent examples including the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and Korean Pharmacopoeia (KP).
- 3)Stability Chamber: Equipment that provides various environmental conditions such as temperature, humidity, and light for stability testing.
Enhancing Supplier Quality
Under its quality management framework, SK bioscience operates a proprietary quality certification program to improve the quality systems of its suppliers across the supply chain. On-site quality assessments are conducted for all Tier 1 suppliers, evaluating manufacturing facilities, production processes, laboratories, and equipment according to relevant regulations and guidelines. For indirect suppliers (Tier 2), systematic quality management is ensured through inspections of Tier 1 suppliers' management systems and regulatory body audits. Regular quality inspections are also performed for all component/raw material suppliers (Tier 3), including excipients and packaging materials.
The Company provides continuous support and monitoring for strengthening the quality systems of its partners through a quality certification program with cycles ranging from 2 to 4 years based on supplier risk assessment grades. Regular quality inspections are conducted according to the Criticality Level (CL) of the raw materials, with quality checks performed on each delivery. For CL 1 materials, comprehensive quality inspections are conducted at least once a year.
IDT Quality Assurance Enhancement
IDT Biologika (hereinafter IDT) holds GMP certifications for various product categories, including human and veterinary medicinal products, vaccines, and more. The company’s certifications include EU GMP, US FDA cGMP, WHO, ISO 9001, and other country-specific quality assurance certifications. Product testings are performed at every stage, from raw materials to starting materials, intermediates, final products, and primary packaging.
IDT’s Quality Oversight system is built around continuous monitoring of all relevant GMP guidelines. New developments are incorporated into the Company's Quality Systems to ensure that all manufacturing activities remain compliant with regulatory requirements. Transparent collaboration with clients is also essential for ensuring compliance with regulatory filings.
Quality Risk Management is a core component of the Quality System and is deeply integrated into internal quality processes. A professional Audit Management Team handles client audits and regulatory inspections. While the approach to quality is universally applicable, it is tailored to meet different regulatory requirements. IDT also supports product release by Qualified Persons (QP) in Europe and Quality Assurance release in the U.S.
Pharmacovigilance (PV)
Pharmacovigilance System
SK bioscience has established a pharmacovigilance (PV) process that spans the entire life cycle of a drug.
Safety information is collected and analyzed from non-clinical and clinical trials in the drug development stage, and based on the results, we identify significant risks to the product and develop a post-market risk management plan.
After the product is marketed, we continue to collect adverse event information from healthcare professionals and recipients to identify safety information that was not identified during the development stage, based on the risk management plan established in advance. The collected information is systematically accumulated in our safety database which is used to evaluate the risk/benefit of the products. Based on the evaluation results, we prepare risk mitigation measures by reflecting them in the product supplements, explanatory materials for physicians and professionals, and patient instructions, and implement proactive and thorough safety management to minimize expected risks.
Pharmacovigilance Training
All employees of SK bioscience receive regular pharmacovigilance training at least once a year to thoroughly manage the quality and safety of the products we produce. New hires are required to complete basic pharmacovigilance training within one month of joining the Company, and all employees should take a pharmacovigilance training annually. Additionally, the Marketing Division responsible for products sales receives an additional training beyond the regular one they receive once a year.
Pharmacovigilance Activities
SK bioscience has advanced its pharmacovigilance system to meet the pharmacovigilance requirements of global regulatory authorities. To make it easier and faster for employees to report safety information on our products to the departments in charge, we have established and are operating a SIRS1), and we have established and are operating a Safety Database to manage the collected information in accordance with international standards.
In addition, we restructured the Pharmacovigilance Division to strengthen the expertise of our PV activities. We have divided the responsibilities of the existing Pharmacovigilance Division into two main areas: planning drug risk management and establishing risk mitigation measures, and conducting risk-benefit assessments based on collected safety information. Through this segmentation and specialization, we strive to continuously advance our pharmacovigilance (PV) work.
In addition, we have entered into separate Safety Data Exchange Agreements with customers who have introduced our products to collect safety information on products, establish, and operate necessary risk mitigation measures in the contracted areas.
- 1)SIRS : Safety Internal Reporting System
Pharmacovigilance System Improvement
SK bioscience conducts the following practices to further advance our pharmacovigilance system.
01. Enhancement of Pharmacovigilance and Monitoring System
Since 2019, We have established and operated the PSMF2), and we regularly update it. Although the PSMF has not yet been legislated in Korea, we have been working to improve the overall level of our PV business system by implementing the PSMF, and we are striving to reflect the requirements of advanced regulatory organizations such as the European Medicines Agency (EMA) in our work. Our Standard Operating Procedures (SOPs) have also been established and operated in line with this philosophy.
02. Operation of Safety Signal Detection Procedures
In May 2023, we established a business process to periodically review the collected data to detect Safety Signals in medicines. Besides the data collected by the Company, we also utilize and analyze raw data collected by the Korea Institute of Drug Safety and Risk Management to monitor unexpected hazards and irregularities.
03. Drug Safety Monitoring System Improvement through Internal and Partner Companies Audits
Starting in 2023, SK bioscience began internal audits of the pharmacovigilance system, and in 2024, we began auditing the pharmacovigilance systems of our partners. Through periodic pharmacovigilance audits, we ensure that our pharmacovigilance business system operates in accordance with relevant regulations, and we examine detailed business systems to ensure that they are efficient and systematic. After the audit, we take action items based on the results of the audit to improve our business procedure, so that both SK bioscience and our partners can continue to enhance the level of product safety management.
- 2)PSMF : Pharmacovigilance Systems Master File
Antimicrobial Resistance (AMR)
As the Company that develops, produces, and sells vaccines, SK bioscience recognizes the critical global public health risk posed by increasing antibiotic resistance. According to the World Health Organization (WHO), antibiotic resistance is among the top 10 global public health threats. Resistance diminishes the efficacy of antibiotics (including antibacterial, antiviral, and antifungal agents), leading to the spread of infections, severe diseases, disabilities, and increased mortality.
Moreover, antibiotic resistance is identified as a threat to global development. The primary cause of antibiotic resistance is the misuse and overuse of antibiotics in humans, animals, and plants. While this is a worldwide issue, it is particularly severe in underdeveloped countries with fragile healthcare infrastructures, causing significant economic burdens on patients.
Preventing disease infections through vaccination is one method to reduce antibiotic misuse. SK bioscience is committed to developing and marketing effective and safe vaccines to protect humanity from diseases. The Company aims to mitigate global vaccine distribution imbalances and indirectly contribute to reducing public health risks associated with antibiotic resistance by implementing a glocalization strategy in regions with insufficient vaccine production capabilities.
Responsible Marketing
Responsible Marketing Policy
Marketing Compliance
SK bioscience conducts responsible marketing in compliance with relevant laws and regulations, including the Pharmaceutical Affairs Act, the Medical Service Act, the Fair Trade Act, and the Code of Fair Competition. To this end, we have established compliance regulations and put in place detailed guidelines for providing economic benefits to healthcare professionals.
In 2023, SK bioscience published and distributed a Fair Trade Compliance Handbook to all employees. This handbook aims to enhance understanding and adherence to laws related to fair trade, the Pharmaceutical Affairs Act, and the Code of Fair Competition. It includes the fundamental principles and guidelines of fair trade, aiming to ensure transparency and fairness in marketing activities.
We also contribute to protecting consumers and creating a fair competition environment in the industry by complying with laws and regulations related to fair trade and strictly regulating activities related to promotion and advertisement. In particular, to prevent false and exaggerated advertisements, we specify the basic principles and scope of advertisement for pharmaceuticals and establish detailed compliance guidelines for quasi-drug advertisement to minimize the possibility of product misuse.
When producing promotional materials used by our Marketing Division, we use an internal review process called the RED system. It allows us to cross-check whether there are any violations from the medical, licensing, and legal perspectives. We do not conduct any marketing or promotion that may induce prescription of off-label products1).
- 1)Off-label Products : Unapproved medical supplies or products
Always-on Monitoring for Responsible Marketing
SK bioscience ensures that our sales partners participate in fair trade by conducting annual monitoring of marketing and promotional activities and obtaining fair trade agreements from them.
To ensure compliance with marketing activities in advance, any expense discussions must be agreed upon by the Compliance Division. In addition, after expenses have been incurred, expense reports are instantly monitored to ensure compliance with the Code of Fair Competition and provide quick feedback to support marketing activities.
When violations are detected during the monitoring process, we assign penalty points and reflect the results in organizational and individual KPIs to encourage responsible marketing activities. In the case of serious violations, we take disciplinary proceedings in accordance with Article 14 (Sanctions against employees) of the Compliance Regulations, the results of which are fully disclosed to our members.
We analyze the causes and consequences of the breaches we find and incorporate the lessons learned into our compliance training to prevent the same incidents from happening again and provide practical lessons learned.
Always-on monitoring process for marketing and promotion activities
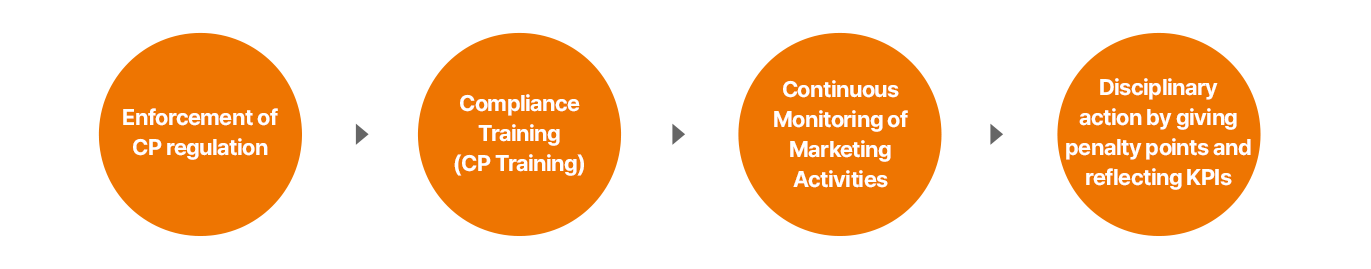
- CP규정 운영
- 준법교육 (CP교육)
- 마케팅 활동 상시 모니터링
- 위반 점수 부여 및 KPI 반영 징계
CP (Compliance Program) Check of Marketing Activities
- 사전 합의:CP 사전 합의 의무, 판촉 활동에 대한 승인
- 보고서 제출:세부 증빙을 포함한 지출내역 보고서를 시스템에 작성·보관
- 모니터링:집행 적정성 모니터링, 모니터링 결과를 개인/조직 KPI 평가에 반영
- 피드백:위반 발생 시 결과 피드백
Marketing Compliance Training
SK bioscience conducts annual training sessions for relevant divisions on compliance with various marketing activities. We strive to establish a fair drug distribution order by providing detailed training on compliance regulations that should be paid attention to in marketing activities, including the Pharmaceutical Affairs Act, the Fair Trade Act, the Code of Fair Competition, and digital marketing as well as how to write an expense report.
2024 Marketing Training Status
| Content | Target | Date |
|---|---|---|
| Marketing CP training (CP regulations, CP KPI standards, Pharmaceutical Affairs Act, Code of Fair Competition, CP violations, etc.) | Marketing & Sales Office |
|
| Training of the Fair Trade Act | Marketing & Sales Office | January 19, 2024 |
Customer Satisfaction Strategy
Vision & Strategy for Customer Satisfaction
Vision
The Company must continuously satisfy customers to gain trust from customers and ultimately develop together with them.
Strategy
- A unified system from licensing to sales
- Strategies specialized for each public and private market
- Portfolio that encompasses infants, children, and adults of all ages
Customer Classification
SK bioscience categorizes its customers into distinct segments, including public and private as well as domestic and international markets for effective management.
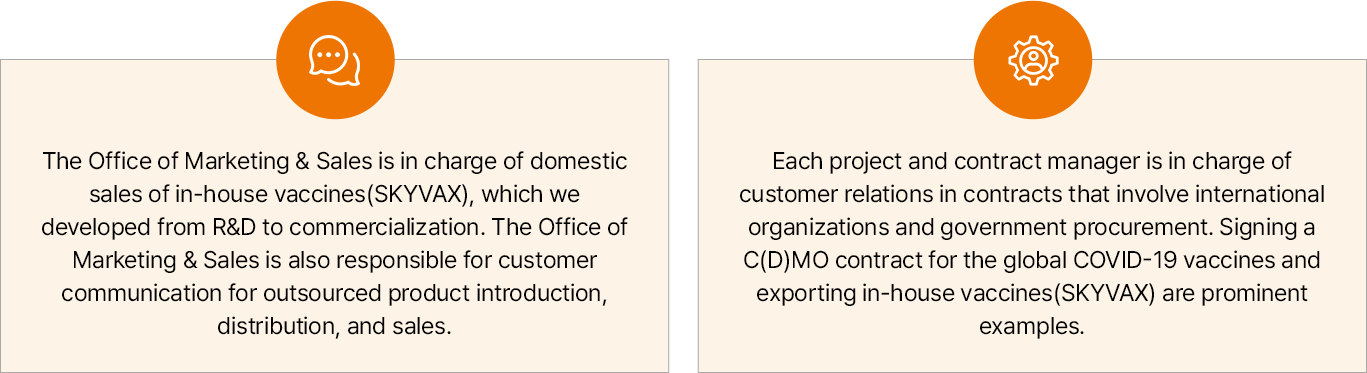

- 연구개발부터 상업화까지 진행한 자체개발 백신(SKYVAX)의 국내 영업, 타사 상품 도입 및 국내 유통판매의 경우 당사의 마케팅 조직에서 고객사 커뮤니케이션을 담당함
- 해외 코로나19 백신의 C(D)MO 계약 체결 및 자체 개발 백신(SKYVAX)의 해외 수출의 경우 국제기구 및 정부 입찰 계약을 통해 제품이 공급되므로 각 프로젝트 및 계약 담당자가 고객을 관리함
Product Information Provision and Consultation
SK bioscience operates a customer consultation service to respond promptly and professionally to consumer inquiries about our vaccine products distributed through general hospitals and clinics. Our professional consultation staffs respond quickly to various customer inquiries such as adverse events and product purchases to minimize consumer anxiety and inconvenience. We also prioritize quality control, safety, accurate information, and maintaining the cold chain throughout the distribution of our vaccines, and transparently communicate key information to our customers, including safety information secured during the approval process. Moving forward, SK bioscience plans to continue to develop marketing strategies to strengthen trust with customers and enhance customer satisfaction, centered on the superior quality, safety, and effectiveness of our products.

Example of Product Information Provision for Customers (SKYVaricella Manual)
Gathering Adverse Event Data
SK bioscience collects confirmed adverse event reports from healthcare professionals and products users through our Customer Contact Center. The collected information is forwarded to our dedicated Pharmacovigilance (PV) Division through our safety information reporting system, and if necessary, we follow up and investigate to obtain additional information.
Domestic Customer Communication
SK bioscience actively communicates with domestic customers through various channels. Our customers include major client companies and general consumers, foreign pharmaceutical companies that import our products, and partners who are authorized to jointly market our products. We have offices in the Seoul Metropolitan area and provinces to facilitate active interactions with customers and regulatory authorities, which are critical when expanding the vaccine business and promoting new businesses.
To actively communicate with key stakeholders, SK bioscience conducts online and offline meetings led by the Steering Committee and a relevant working-level group. Executives and the working-level group from each company meet periodically to discuss major marketing plans for each product, as well as quarterly, monthly, and annual plans, as outlined in their respective contracts.
