Double Materiality Assessment
Double Materiality Assessment
SK bioscience performs annual materiality assessment to identify material topics in our ESG management and reflect them in our ESG strategies and decision-making. In 2025, we conducted a double materiality assessment in accordance with the European Financial Reporting Advisory Group’s (EFRAG) Materiality Assessment Implementation Guidance to enhance reliability of the assessment and alignment with international standards. In addition, we reflected principles for selecting reporting topics in the European Sustainability Reporting Standards (ESRS) and Global Reporting Initiative (GRI) Standards to ensure systematization and objectivity in our assessment.
The double materiality assessment is a process that comprehensively analyzes sustainability topics from two perspectives, and ultimately determines material topics given those perspectives. Those are impact materiality assessment analyzing the impacts of a company’s business activities on environment and society, and financial impact assessment examining the potential financial risks and opportunities caused by sustainability topics. Through this, SK bioscience aims to understand key stakeholders’ expectations and regulatory requirements and establish strategies to create a long-term corporate value.
Double Materiality Assessment Process
Step 1. Identifying Topic List
To understand the Company’s sustainability topics, SK bioscience considered business activities across the value chain and both internal and external stakeholders and reviewed the previous year’s sustainability topic list. In addition, we comprehensively analyzed domestic and international peer review and benchmarking, global ESG ratings and disclosure standards, and global initiatives to identify key sustainability topics in bio industry. Through this process, a total of 25 sustainability topics were determined.
Step 2. Analyzing Impact, Risk, and Opportunity
SK bioscience conducted various metrics-based analysis to identify the impacts, risks, and opportunities of 25 sustainability topics.
Impacts, Risks, and Opportunities
| Category | Details |
|---|---|
| Impact Factors |
|
| Risk and Opportunity Factors |
|
Step 3. Assessing Double (Impact & Financial) Materiality
A total of 25 sustainability topics were analyzed for their impact, risk and opportunity factors to assess impact materiality and financial materiality.
Double Materiality Assessment (Stakeholder Survey)
| Category | Impact Materiality* | Financial Materiality |
|---|---|---|
| Target |
|
|
| Criteria |
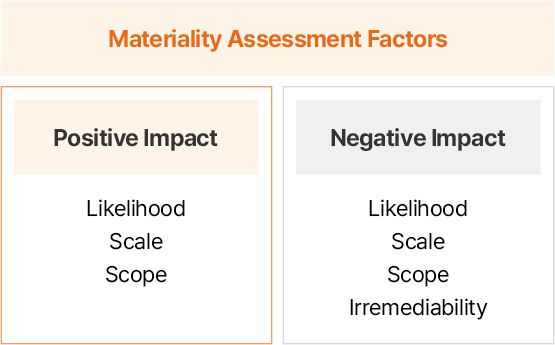

|
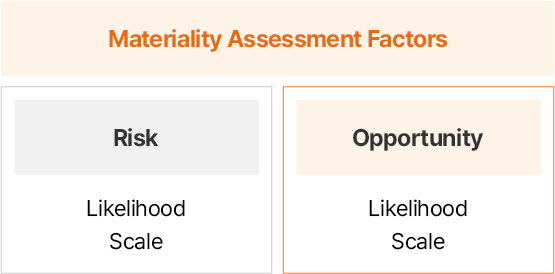

|
- *Evaluated impact areas by ‘own operations’ and ‘up/downstream’
Step 4. Determining the Double Materiality Assessment Results
Based on double materiality through various metrics analysis and stakeholders' survey, we prioritized five impact material topics and three financial material topics. The five impact material topics are: 1) Talent Acquisition and Professionals Development, 2) Strengthening Product Safety and Quality, 3) Enhancing Access to Healthcare, 4) Hazardous Chemicals and Pollutants Management, and 5) Implementing Ethical Compliance Management. The three financial material topics are: 1) enhancing access to healthcare, 2) enhancing product safety and quality, and 3) talent acquisition and professionals development.
SK bioscience discloses detailed information on all sustainability topics, including impact and financial material topics, through this ESG website. In addition, material topics were finalized through the Board-level review, and targets and performance are transparently disclosed by aligning them with our management and ESG strategies.
Sustainability Topic List
- ● Impact material topic
- ● Financial material topic
| E (Environmental) |
|---|
| Hazardous Chemicals and Pollutants Management ● |
| Action on Climate Change |
| Improving Resource Efficiency and Recycling |
| Strengthening Management of Water Resources |
| Conducting Biodiversity Conservation Activities |
| Minimizing Products’ Lifecycle Environmental Impact |
| Managing Environmental Management Goals and Performance |
| S (Social) |
|---|
| Talent Acquisition and Professionals Development ● ● |
| Strengthening Product Safety and Quality ● ● |
| Enhancing Access to Healthcare ● ● |
| Promoting Human Rights Management and Diversity |
| Practicing Customer Satisfaction Management |
| Enhancing Workplace Safety and Health of Employees |
| Building a Sustainable Supply Chain |
| Information Security Management |
| R&D and Clinical Trial Ethics Compliance |
| Ethical Marketing and Consumer Rights Protection |
| Expanding Corporate Philanthropic Activities and Community Value Creation |
| Promoting Employee Happiness |
| G (Governance) |
|---|
| Implementing Ethical and Compliance Management ● |
| Enhancing Transparent/Responsible Management centered on the Board |
| Transparent Tax Management and Payment |
| Building Sustainable Innovation and Market Leadership |
| Strengthening Stakeholders Communication and Disclosure |
| Integrated Management of Financial and Non-financial Risks |

| Priority | Impact Material Topics (5) | 2024-2025 Ranking Change |
|---|---|---|
| 1 | Talent Acquisition and Professionals Development | ▲ 9 |
| 2 | Strengthening Product Safety and Quality | ▲ 1 |
| 3 | Enhancing Access to Healthcare | ▲ 10 |
| 4 | Hazardous Chemicals and Pollutants Management | ▲ 2 |
| 5 | Implementing Ethical and Compliance Management | ▼ 1 |
| Priority | Financial Material Topics (3) | 2024-2025 Ranking Change |
|---|---|---|
| 1 | Enhancing Access to Healthcare | ▲ 12 |
| 2 | Strengthening Product Safety and Quality | ▲ 1 |
| 3 | Talent Acquisition and Professionals Development | ▲ 7 |
* While 2024 material topics were eleven in total, including 1) securing mid- to long-term growth engines, 2) embedding ESG into the Company, 3) strengthening product safety and quality, 4) implementing ethical and compliance management, and 5) action on climate change, this year we focused on five impact material topics and three financial material topics, in line with our enhanced materiality assessment process and the level of material topic management. 'Enhancing access to healthcare' was added as a new material topic reflecting the nature of the industry and business environment, while ‘talent acquisition and professionals development,’ ‘hazardous Chemicals and pollutants management,’ ‘strengthening product safety and quality,’ and ‘implementing ethical and compliance management’ were selected as material topics again this year.
Impact Material Topics
SK bioscience selected five material topics with significant environmental and social impacts across the value chain. To analyze the impact of each topic, we defined the relevant value chain areas and key stakeholders, and evaluated the time horizon (short, medium, and long-term) and impact aspects (positive and negative). Based on this, we have established impact management strategies and presented mid- to long-term goals and performance outcomes, which are strengthening ESG management practices linked to our business.
Impact Material Topics
| Priority | Topic | Impact Materiality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Impact Area in Value Chain | Key Stakeholders | Time Horizon1) | Impact Aspects1) | |||||||
| Short | Mid | Long | Positive | Negative | ||||||
| ① | Talent Acquisition and Professionals Development | Worksites, Supply Chain | Employees | ◐ | ● | ○ | ● | ○ | ||
| ② | Strengthening Product Safety and Quality | Worksites, Supply Chain, Product/Service | Customers, Business Partners, Shareholders/Investors | ● | ◐ | ○ | ● | ○ | ||
| ③ | Enhancing Access to Healthcare | Worksites, Supply Chain, Product/Service | Customers, Local Communities | ◐ | ◐ | ● | ● | ○ | ||
| ④ | Hazardous Chemicals and Pollutants Management | Worksites, Supply Chain | Employees, Business Partners, Local Communities | ● | ◐ | ◐ | ● | ◐ | ||
| ⑤ | Implementing Ethical and Compliance Management | Worksites, Supply Chain | Employees, Business Partners, Shareholders/Investors | ● | ◐ | ◐ | ● | ○ | ||
1) ●: High, ◐: Medium, ○: Low
① - Talent Acquisition and Professionals Development
Business Context
To become a global vaccine and biotechnology company, SK bioscience has established Talent Database (TDB) and conducted customized recruitment for prospective job seekers. We also provide various training programs to improve employees' practical competencies and strengthen their professionalism. SK bioscience's efforts to secure and foster experts in vaccine and bio industry will contribute to the development of a sustainable domestic and global bio industry ecosystem.
Impact Management Strategy
SK bioscience operates a global talent recruitment program and implements workforce development programs under the strategy of securing talent and fostering professional manpower.
| ‹Mid- to Long-term Target› | ‹2024 Performance› |
|---|---|
|
|
② - Strengthening Product Safety and Quality
Business Context
SK bioscience produces, manages, and sells pharmaceutical products in a GMP (Good Manufacturing Practices) environment at home and abroad based on a quality management system (QMS). We also strive to strengthen the safety and quality of our products by conducting clinical research and trials, quality assurance, and pharmacovigilance throughout the entire life cycle of pharmaceutical products based on the regulations and guidelines of the Ministry of Food and Drug Safety and each country in which we operate. Strict quality and safety management for biopharmaceuticals, which are directly related to life, leads to customer reliability, and in the long run, increases resource efficiency to minimize unnecessary consumption of resources or raw materials.
Impact Management Strategy
SK bioscience contributes to the creation of a sustainable public health system by enhancing product safety and quality through a rigorous Quality Management System (QMS) across all processes, from drug development to customer purchase.
| ‹Mid- to Long-term Target› | ‹2024 Performance› |
|---|---|
|
|
③ - Enhancing Access to healthcare
Business Context
SK bioscience is committed to improving public health in low- and middle-income countries (LMICs) through participation in multilateral procurement markets of international organizations and implementation of the SKYShield strategy based on 'glocalization' business. We aim to address health inequalities in LMICs by expanding access to healthcare and contribute to the long-term reduction of social costs and strengthening of public healthcare systems through early prevention of disease.
Impact Management Strategy
SK bioscience is involved in several projects and research aimed at creating a global bio-ecosystem to address the global vaccine supply imbalance.
| ‹Mid- to Long-term Target› | ‹2024 Performance› |
|---|---|
|
|
④ - Hazardous Chemicals and pollutants Management
Business Context
SK bioscience strives to recycle and reuse resources used in all production processes of products. In line with the tightening of global regulations, we have advanced the hazardous chemicals management system and utilized our own wastewater treatment facilities to effectively reduce the risk of water pollution. Protecting the health and environment of our communities and customers is top priority, and enhancing the pollutants and hazardous chemicals management can boost customer trust and lead to long-term brand value.
Impact Management Strategy
We have established an environmental management system (EMS) in accordance with ISO 14001 standards and conduct annual compliance assessment for a total of 12 environmental laws including the Water Environment Conservation Act and the Clean Air Conservation Act to identify environmental risks and opportunities. We also strive to minimize environmental impacts, including zero environmental pollution and chemical leakage accidents.
| ‹Mid- to Long-term Target› | ‹2024 Performance› |
|---|---|
|
|
⑤ - Implementing Ethical and Compliance Management
Business Context
SK bioscience's ethics and compliance management goes beyond mere legal obligations and creates various social values such as supplying safe medicines, securing research credibility, creating a fair industrial environment, and building social trust. To this end, SK bioscience has established the Code of Ethics and ethical management principles based on the belief that 'transparent management practices are the beginning of true sustainable management.' We operate an ethics and compliance management system based on the Code of Ethics Practice Guidelines that considers various stakeholders, provide a reporting channel for unethical behavior, and undertake independent audits to ensure transparent management practices.
Impact Management Strategy
SK bioscience has established and operated ethical management-related regulations and guidelines to prevent risks in advance and internalize ethical practices in terms of Prevent-Detect-Respond.
| ‹Mid- to Long-term Target› | ‹2024 Performance› |
|---|---|
|
|
Financial Material Topics
SK bioscience selected three financially material topics that have a significant impact on financial performance, including assets, revenue, earnings, and cash flows. In the analysis of each topic, we carefully considered the areas of impact in terms of cost, revenue, and risk, and reviewed the time horizon (short, medium, and long-term) of impact and the aspects (risks/opportunities). We report our detailed strategies and performance by topics under the main ESG disclosure framework (governance, strategy, risk management, metrics and targets) and plan to actively consider them in management strategy.
Financial Material Topics
| Priority | Topic | Financial Materiality | |||||
|---|---|---|---|---|---|---|---|
| Financial Impact Areas | Time Horizon1) | Impact Aspects1) | |||||
| Short | Mid | Long | Risk | Opportunity | |||
| ① | Enhancing Access to Healthcare |
|
○ | ◐ | ● | ○ | ● |
| ② | Strengthening Product Safety and Quality |
|
● | ● | ○ | ◐ | ◐ |
| ③ | Talent Acquisition and Professionals Development |
|
◐ | ◐ | ◐ | ○ | ● |
1) ●: High, ◐: Medium, ○: Low
① - Enhancing Access to Healthcare
Governance
To improve global public health and reduce vaccine supply imbalances, we regularly review and oversee the issue of expanding access to healthcare at the Board of Directors and ESG Committee levels. Major investments, global vaccine partnerships, and fund contributions are reviewed through internal decision-making process.
Strategy
SK bioscience is working with various national and global organizations to expand access to vaccines to improve global public health and reduce disparities. We are responding to diseases with a high public health burden, such as influenza, zoster, varicella, and typhoid, through our self-developed vaccine portfolio, SKYVAX, and continue to expand global supply, focusing on low- and middle-income countries (LMICs). We are also promoting the SKYShield strategy to respond to the imbalance in the global vaccine supply chain, enhancing vaccine self-reliance capabilities in health-underserved regions such as Thailand through a 'glocalization' model that transfers R&D and production technologies to the local level.
- Opportunity: Reducing healthcare financial costs and gaining access to new markets in LMICs
Risk Management
SK bioscience operates the Investment Committee in case of new projects or investments to expand access to healthcare, which comprehensively deliberates and reviews strategic suitability, investment cost-effectiveness, risks, and ESG impacts. The committee deliberated and reviewed various issues to enhance access to healthcare, including participation in multilateral procurement led by international organizations and expansion of SKYShield strategy target countries.
Metrics and Targets
- Continued expansion of supply to international organizations such as PAHO and UNICEF
- Continued expansion of vaccine supply countries
- Enhancing product quality control
② - Strengthening Product Safety and Quality
Governance
SK bioscience systematically conducts product safety and quality management throughout the entire drug lifecycle in strict compliance with the guidelines of the Ministry of Food and Drug Safety and regulatory agencies in each country. In addition, based on internal audits, quality audit system of business partners, and QA operation systems, we have advanced a risk prevention-oriented quality management system that covers all stages from product design to production and shipment.
Strategy
SK bioscience has established a quality system based on the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and global standards to continuously enhance the safety and quality reliability of pharmaceutical products. We apply GMP and quality standards to the entire process of production and distribution of clinical and commercial products at home and abroad and are continuously enhancing quality operation system by managing and supervising critical processes on-site, effectively managing process deviations, and ensuring data integrity.
- Opportunity: Increasing brand value and sales by improving product reliability
- Risk: Product safety and quality control failures resulting in product recalls and loss of customer trust
Risk Management
SK bioscience operates a pharmacovigilance (PV) system and safety signal detection procedures to proactively manage quality and safety risks that may occur at all stages of the drug life cycle. Based on adverse event reports collected from medical professionals, we analyze the signals and continuously monitor the occurrence of adverse events using post-marketing quality data. Based on this information, we proactively prevent quality issues through organic collaboration between the QA/QC and the manufacturing departments.
We identify supply chain risk, CMO quality issues, and non-compliance risk in advance to establish response strategies, and we systematically record and manage major quality issues such as product recalls, customer complaints, and adverse events through the QMS (Quality Management System).
Metrics and Targets
- Enhancing suppliers’ training and inspection to improve supplier quality and safety management
- Establishing and operating a drug recall system for efficient management of recall drugs
③ - Talent Acquisition and Professionals Development
Governance
SK bioscience checks the efficiency of its workforce operations through CEO reporting and executive meetings and makes decisions on fostering experts and strategic staffing for key projects.
Strategy
We operates a system that predicts human resource needs in advance to secure excellent talents, recruits and retains the right people at the right time, and continues to operate customized training programs for each job and position through the 'SKBS Academy' so that new recruits can quickly adapt and grow in the organization.
- Opportunity: Recruiting and fostering experts to accelerate a sustainable innovation
Risk Management
At SK bioscience, we recognize that proactively securing talented people and enhancing their job skills directly contributes to the Company's growth and productivity. By securing talented people with expertise and execution skills at an earlier stage, we can speed up the development of high-value-added R&D projects and accelerate sales growth by shortening the timeline for new vaccine launches and increasing the likelihood of technology commercialization. In addition, we expect that global vaccine experts trained through partnerships with global vaccine education cooperation systems (WHO, IVI, etc.) will lead to the expansion of our vaccine exports, technology transfer projects, and joint R&D opportunities.
Metrics and Targets
- Establishing mid- to long-term strategy of recruiting and expanding talent pool for acquiring professionals
