SHE Management System
SHE Management System & Policy
SK bioscience has established the SHE Management Policy, which states that "protect human life first, preserve the environment, and finally safeguard the Company's assets and services". This policy applies equally not only to our employees but also to all members of our suppliers, outsourcing partners, domestic and overseas entities for production and sales, subsidiaries, sub-subsidiaries and joint ventures. Additionally, all stakeholders of SK bioscience are encompassed in our SHE implementation activities, including the local community and the business ecosystem.
Based on this SHE Management Policy, we strive to realize an accident-free workplace by complying with relevant laws and standards, improving risk factors, and creating a voluntary safety culture, and to become a company trusted and supported by society.
SHE (Safety·Health·Environment) Management System
SK bioscience has established the SHE (Safety·Health·Environment) Management System, recognizing that safety, health, and environment are integral components of our management principles of promoting human health. The SHE management system is applied to workers’ duties, scope of activities, and all types of resources, products, and service activities handled by workers. We consider it the cornerstone of our management activities and strive to pursue the happiness of all our stakeholders.
Misson
Elevating SHE infrastructure to drive BS 3.0 success & Establishing a sustainable SHE management system
Vision
SHE Leading Company in Pharmaceutical/Bio industry
SHE Management System
- Safety (안전) : 사고예방을 통해 근로자의 생명 및 설비를 안전하게 보호
- Health (보건) : 위험물질에 의한 중독,질병 예방 및 건강증진
- Environment (환경) : 대기, 수질, 폐기물 및 토양오염 방지 및 환경영향 최소화

ISO 45001 Certification
SK bioscience operates a safety and health management system based on ISO 45001, the international standard for safety and health management system. Andong L HOUSE acquired the OHSAS 18001 and KOSHA 18001 safety and health management certifications in 2016 and has maintained certification after transitioning to ISO 45001 in 2018. Meanwhile, the HQ and R&D Center in Pangyo are systematically managed in accordance with our SHE operation policy to prevent industrial accidents. IDT also plans to obtain ISO 45001 on occupational health and safety management system in 2025.
SHE Management Goals
SK bioscience endeavors to minimize the impact of its business activities on employees, stakeholders, local communities, and the environment by establishing SHE management goals. Accordingly, we strive to prevent SHE accidents at our worksites, comply with relevant laws and regulations, and comprehensively manage our safety, health, and environmental impacts. Moreover, in accordance with the operating regulations of the Board, we review the annual safety and health plan and report to the Board of Directors on a regular basis at the beginning of each year.
SHE Goals
- 1Establishing a safe and healthy workplace for employees and external stakeholders
- 2Reducing environmental impact across all stages of business operations and product life cycles
- 3Promoting a culture of Safety, Health, and Environment (SHE) that involves all employees
SHE Management Mid- to Long-Term Goals
SK bioscience strictly complies with safety and health guidelines at all workplaces and prioritizes the prevention of all accidents. Particularly, we have set the goal of achieving 'zero fatal accidents' and established a mid- to long-term roadmap to achieve this goal. To this end, quantitative targets have been set for the number of major accidents, fatalities, and the Lost Time Injury Rate (LTIR), and we are implementing improvements step by step through annual action tasks.
| Category | 2024 Performance |
Goal | ||||
|---|---|---|---|---|---|---|
| 2025 | 2026 | 2027 | 2028 | 2029 | ||
| Target | LTIR 0.090 | Fatal Accidents: Zero LTIR 0.081 |
Fatal Accidents: Zero LTIR 0.073 |
Fatal Accidents: Zero LTIR 0.066 |
Fatal Accidents: Zero LTIR 0.059 |
Fatal Accidents: Zero LTIR 0.053 |
| Task | Enhancing incident prevention system and execution | Utilizing SKBS SHE Culture & Reinforcing vulnerabilities | Embedding SHE competencies & Enhancing proactive management | Building sustainability through employees’ engagement | Establishing a company-wide ISO system | Completing 'field-centered SHE' that reflects SKBS characteristics |
| Detail |
|
|
|
|
|
|
SHE Management Governance
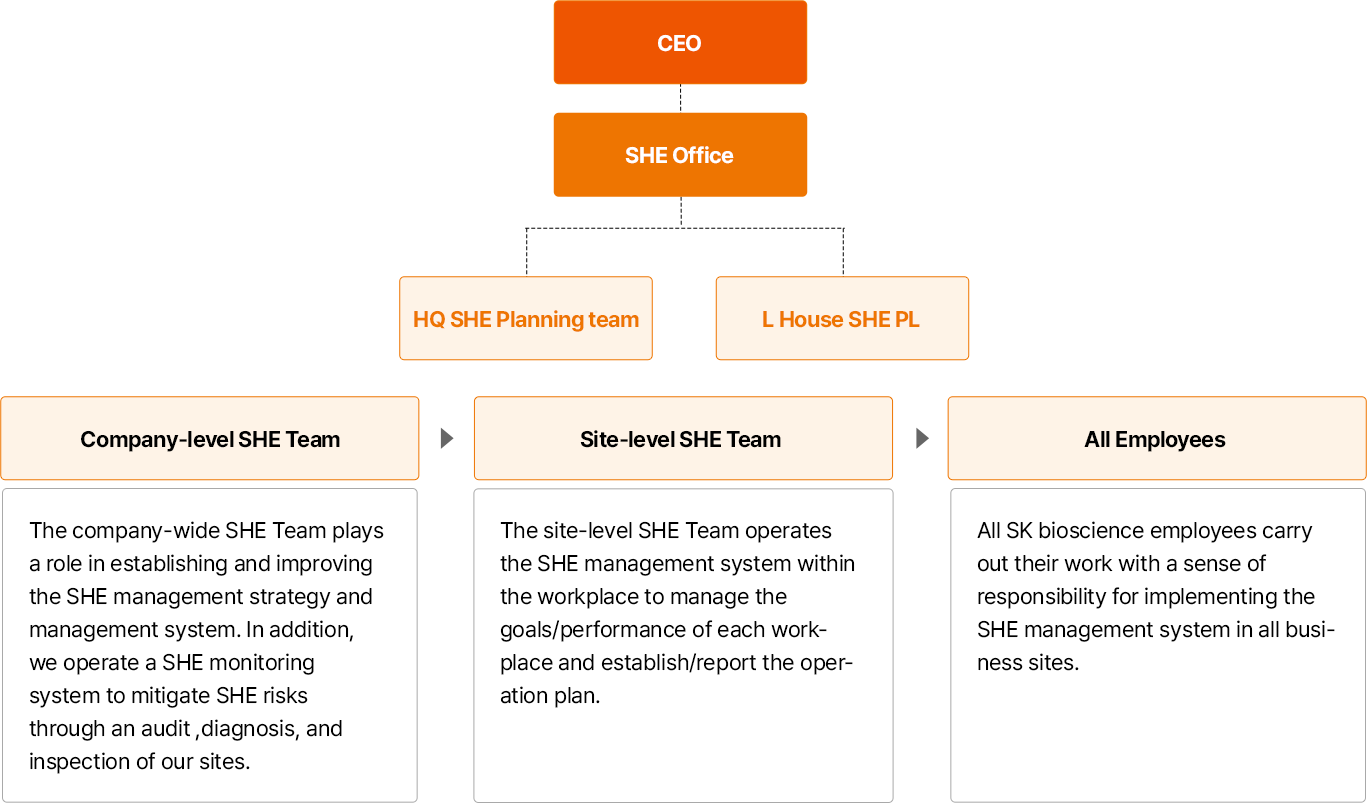

- CEO
- SHE담당
- 본사 SHE기획팀 , L HOUSE SHE PL
- 전사 SHE 전담조직 : SHE 전담조직은 SHE 경영전략과 관리체계를 구축하고 개선하는 역할을 수행합니다. 또한, SHE 모니터링 체계를 운영하여 감사(Audit), 진단, 점검을 통한 사업장 이슈 개선 및 SHE 리스크 관리를 실시하고 있습니다.
- 사업장 SHE 전담조직:사업장 SHE 전담조직은 사업장 내 SHE 관리체계 운영에 따른 사업장 단위 목표와 성과를 관리하며 운영계획의 수립 및 보고를 수행하고 있습니다.
- 전 구성원:SK바이오사이언스의 모든 구성원은 전 사업장 SHE 경영시스템 실천에 대한 책임의식을 가지고 업무를 수행하고 있습니다.
Workplace Safety & Health Management
Occupational Safety and Health Committee
SK bioscience has established a joint Occupational Safety and Health Committee between labor and management at the HQ and Andong L HOUSE, respectively, for the health of workers and accident prevention at the worksite. The Occupational Safety and Health Committee is held on a quarterly basis. It listens to the opinions of workers on changes to major safety and health regulations, including the basic safety and health management system, and strives to reflect them in practice.
In addition, we are seeking improvements and follow-up measures that can effectively protect the safety of workers by ensuring an equal number of representatives from both the employer and employee sides in the committee. The results proposed and progressed in the committee are being shared at the next meeting for further review.
Operational Status of Occupational Safety and Health Committee by worksites
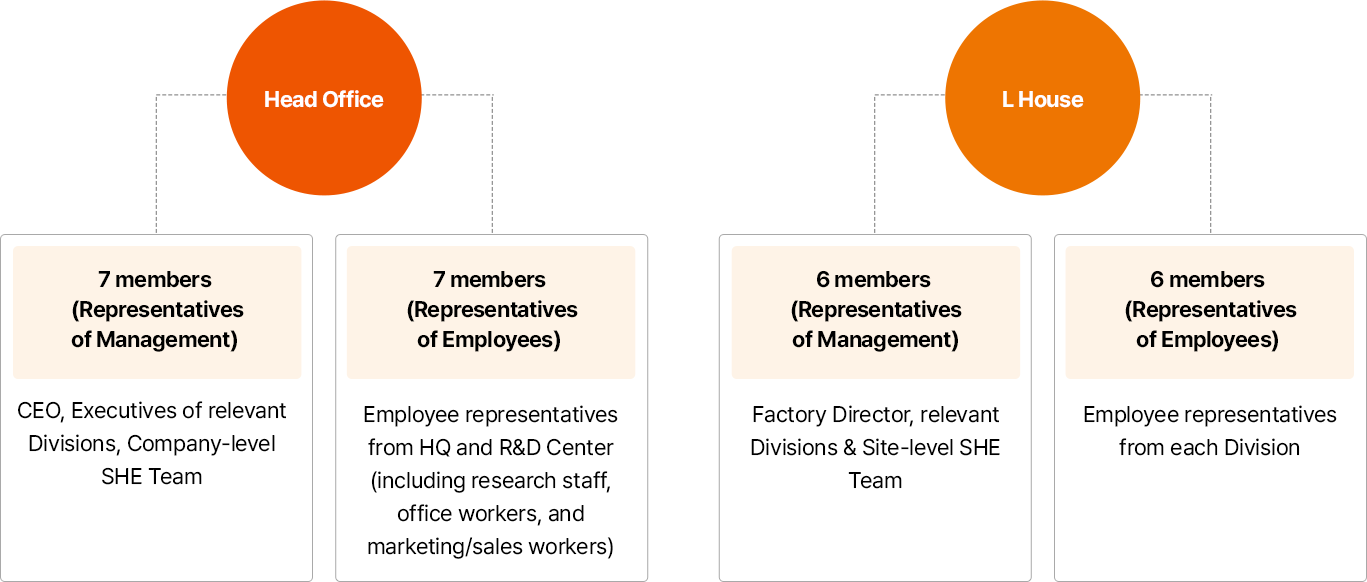
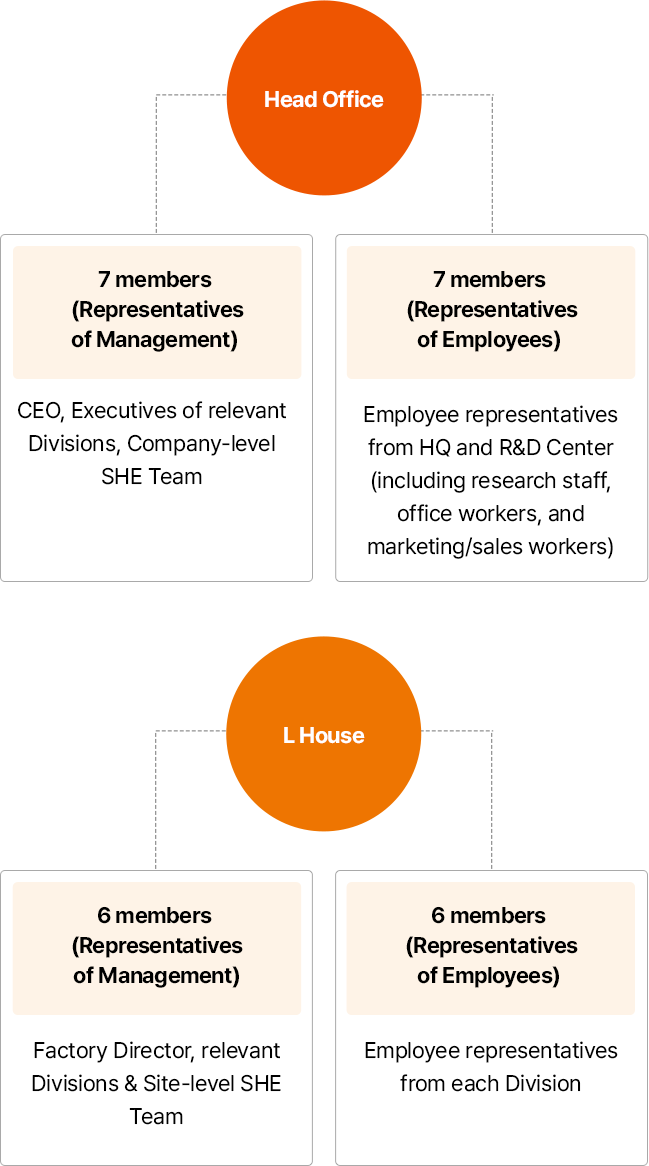
- 본사
- 사용자 측 7명:CEO, 유관 부서 임원, 전사 SHE 전담조직
- 근로자 측 7명:근로자 대표, 본사 및 R&D 센터(연구직, 사무직, 마케팅/영업직 포함)
- L HOUSE
- 사용자 측 6명:공장장, 유관 부서, 사업장 SHE 전담조직
- 근로자 측 6명:부서별 근로자 대표
Main Agenda Items of Occupational Safety and Health Committee
- 1Setting SHE priority projects and goals
- 2Safety and health issues raised by SHE officials and workers
- 3Developing and revising safety and health management regulations
- 4Safety and health issues related to the introduction of hazardous machinery and other facilities
- 5Safety and health training for workers
- 6Conducting inspections and improving the working environment, including measuring the working conditions
- 7Health management of workers, including health check-ups
- 8Investigating the causes of serious accidents and establishing measures to prevent recurrence
- 9Recording and maintaining statistics on industrial accidents
- 10Implementing and promoting the safety and health management system
- 11Other important matters related to SHE
Safety & Health Risk Assessment Process
SK bioscience specifies the removal of SHE risk factors in a significant process of implementing the management system and conducts risk assessments for all operations to proactively identify and prevent accidents. We hereby discover all potential risks that can arise from operations, and secure safety and health measures, so that workers can work in a safe environment.
The risk assessment begins with a preliminary step to clearly identify the objectives and scope of the assessment, followed by the identification of hazardous risk factors and a task-specific assessment to isolate high-risk tasks. For each risk factor, the risk level is then evaluated by considering the likelihood and severity of injury or illness and determining whether it is acceptable. For unacceptable risks, we establish and implement measures to reduce them, and manage safety and health risks through continuous monitoring and improvement activities.
In 2024, based on the results of the risk assessment, we continued to identify and improve various risk factors that may occur during work. Particularly, we actively promoted the improvement of safety facilities to eliminate harmful factors, and made efforts to create a healthier work environment, such as manufacturing and supplying customized insoles to reduce workers' fatigue caused by standing for long periods of time.
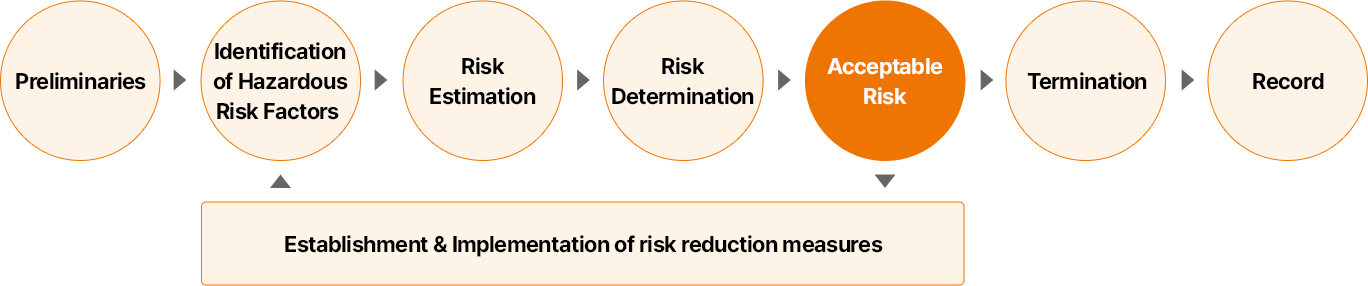
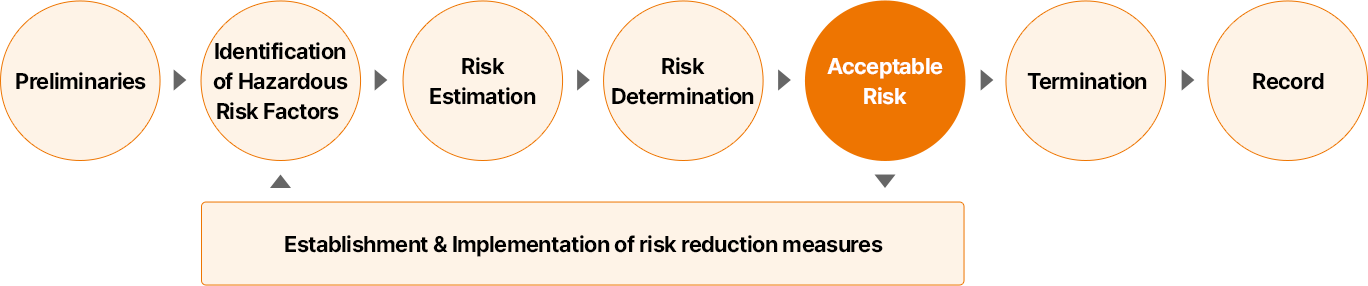
- 사전 준비
- 유해 위험 요인 파악
- 위험성 추정
- 위험성 결정
- 허용 가능 위험성
- 종료
- 기록
위험성 감소 대책 수립 및 시행
On-site Work Hazard Management
To protect the health of our employees, SK bioscience regularly undertakes hygiene and work risk assessment in the workplaces, and conducts proactive health management to prevent occupational diseases. Through this, we identify health risk factors such as hazardous chemicals at an early stage and minimize risks exposure through systematic measures. Especially we have developed a chemical management system (CMS) that can monitor the entire process of chemicals from warehousing to disposal in real time to reduce potential risks from chemical exposure through proactive risk assessment and usage management.
We also conduct preventive management for musculoskeletal disorders through risk assessment. Regular medical examinations are conducted to monitor workers for any health abnormalities. Additionally, for employees who handle or are exposed to specific hazardous factors, we conduct specialized medical examinations targeting those specific hazards. As a result of this integrated health management system, there were no major health incidents including fatal injuries or occupational diseases in 2024.
On-site Hazardous Chemical Risk Assessment
| Categorization of On-site Hazardous Factors | Implementation of Risk Assessment | Major Improvement Activities |
|---|---|---|
| Hazardous risk factors causing serious injuries1) |
|
|
| Hazardous risk factors causing serious diseases2) |
|
|
- 1)Injuries resulting from contact with chemicals between tasks and fire caused by using hazardous materials, etc.
- 2)Diseases resulting from exposure to hazardous chemicals between tasks, musculoskeletal strains caused by handling heavy materials, etc.
Establishment of Safety Culture
Establishment of a Safe Work Environment
SK bioscience conducts SHE joint inspections every month with the participation of each team leader to enhance management's engagement in SHE activities. In addition, we closely monitor direct and visible accidents, as well as risk factors including near misses, in the workplace to ensure that no small risk factors go unnoticed.
In the event of accidents, we have an accident investigation process in place to ensure immediate reporting of workplace-related incidents. Moreover, we actively work towards establishing a safe working environment by forming accident investigation committees that vary in accordance with the severity of the accidents. These committees identify the root causes of the accidents and establish measures to prevent their recurrence.
Employee’s Right to Suspend Work
SK bioscience protects the right of workers to suspend work in accordance with the Safety and Health Management Regulation. The Regulation states that workers can stop work and evacuate if they identify a potential risk of industrial accidents, and will not face any penalties for doing so.
- 1Where imminent danger of an industrial accident exists, or a serious accident has occurred, the Company shall take necessary measures for health and safety, such as the immediate suspension of operations and evacuation of employees from the place of business, after which work may resume.
- 2Where any employee suspends work and evacuates due to any urgent risk of an industrial accident, he/she shall promptly report it immediately to the superior officer, who shall take appropriate measures to address the situation.
- 3Where reasonable grounds exist to believe that any imminent danger of an industrial accident exists, a business owner shall not dismiss or give other disadvantages to employees by reason of their suspending work and evacuating pursuant to paragraph (2).
Safety Culture Assessment
SK bioscience improves the SHE system at worksites through objective evaluations by inspecting SHE activities with the affiliated companies. Starting from 2022, we have implemented SHE activities evaluations using a checklist. Also, we evaluate the level of our safety culture and address any shortcomings by actively engaging with other business sites of affiliated companies and benchmarking them.
We also measure and verify the effectiveness of our worksites' safety and health management system through annual assessments of the system and semi-annually assessments of legal compliance. In addition, once a year, we verify the effectiveness of the system through ISO 45001 post/renewal audits, an international standard certification.
Safety Culture Training
SK bioscience conducts regular SHE trainings for all employees. Trainings were provided on risk assessment based on hazard characteristics, advanced safety case studies, and Lock Out Tag Out (LOTO) procedures. Furthermore, various other training sessions, including those for partner companies, were conducted to enhance safety awareness. We are committed to promoting a safety culture by offering various training programs to all employees and suppliers, raising awareness about the significance of occupational safety and health management.
| Category | Content |
|---|---|
| Regular SHE Training |
|
| Additional Training |
|
| Visitor/Partner Training |
|
Enhancing Executive SHE Engagement
SK bioscience has been operating the 'Executive SHE See & Do' program since March 2024 to strengthen safety, health, and environment (SHE) leadership. As a part of the program, the CEO personally visits major worksites such as Pangyo HQ and R&D Center, Andong L HOUSE, and Songdo Global R&D Center to inspect sites with site managers and host safety talks with employees to share SHE issues and seek ways to improve them.
The program drives continuous improvement activities and embeds safety culture in the SHE environment and this is a key leadership activity for creating a safe and healthy work environment.

Pangyo R&D Center

Andong L HOUSE

Songdo Global R&PD Center construction site
Communication Channels for Safety
SK bioscience is creating a culture of communication for safety by opening channels for employees to freely express their opinions on hazardous factors and environmental risks that may arise in the workplace. In May 2023, SK bioscience launched the ‘SHE! Say!’ program, empowering employees to proactively identify and mitigate SHE-related risks.
Additionally, we executed Set-play1) activities to minimize risks by detecting blind spot hazards and training employees on their specific roles in emergency situations.
- 1)Activities that identify the risks of a task in advance and then train each person on their role in the event of an emergency
'SHE Say' Reports and Improvements
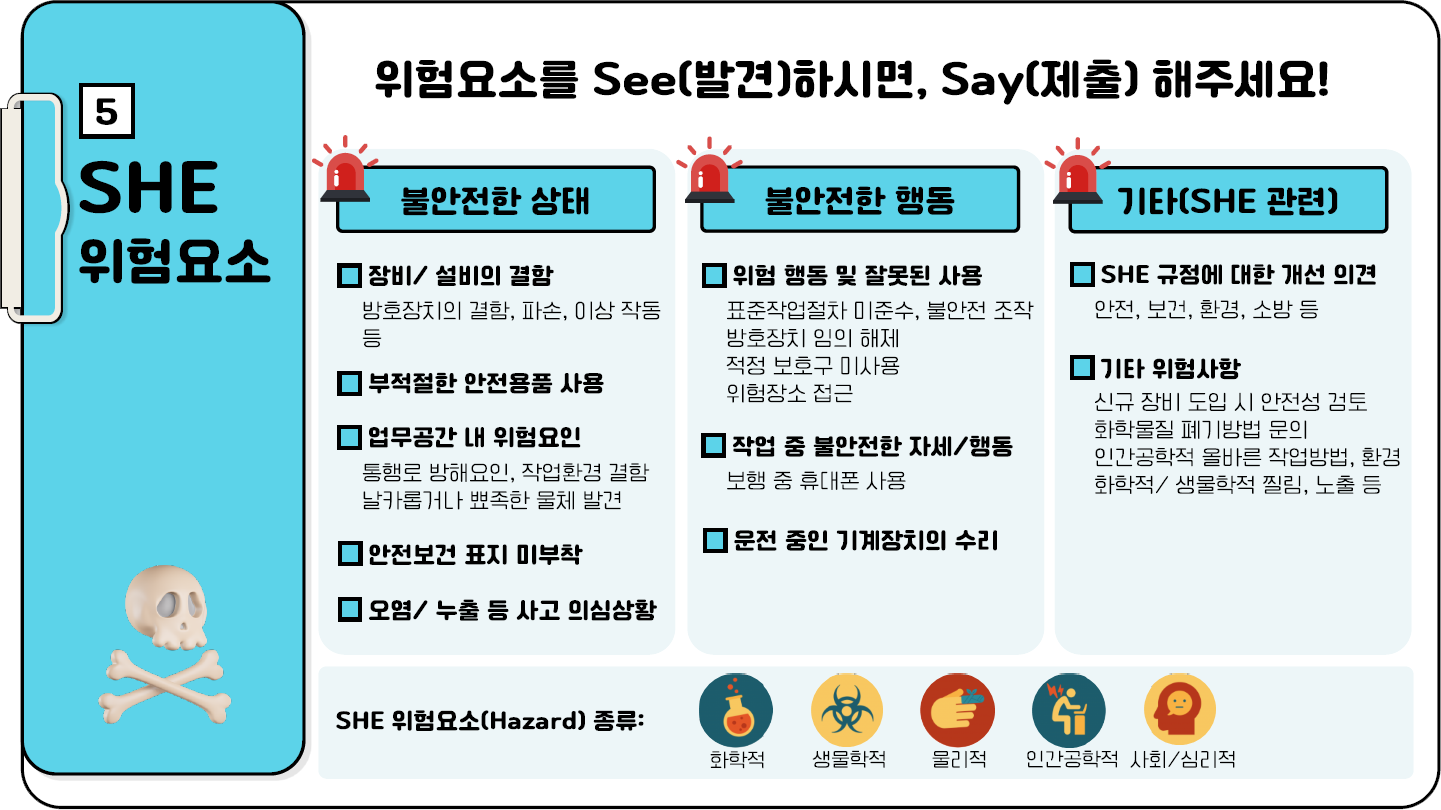
SHE위험요소
위험요소를 See(발견)gktlaus, Say(제출) 해주세요
-
- 불안전한 상태
- 장비/설비의 결함 : 방호장치의 결함, 파손, 이상 작동등
- 부적절한 안전용품 사용
- 업무공간 내 위험요인 : 통행로 방해요인, 작업환경 결함 날카롭거나 뾰족한 물체 발견
- 안전보건 표지 미부착
- 오염/누출 등 사고 의심상황
-
- 불안전한 행동
- 위험 행동 및 잘못된 사용 : 표준작업절차 미준수, 불안전 조작 , 방호장치 임의 해제, 적정 보호구 미사용, 위험장소 접근
- 작업 중 불안전한 자세/행동 : 보행 중 휴대폰 사용
- 운전 중인 기계장치의 수리
-
- 기타(SHE 관련)
- SHE 규정에 대한 개선 의견:안전, 보건, 환경, 소방 등
- 기타 위험사항:신규 장비 도입 시 안전성 검토, 화학물질 폐기방법 문의, 인간공학적 올바른 작업방법, 환경, 화학적/생물학적 찔림, 노출 등
-
- SHE 위험요소(Hazard)종류
- 화학적
- 생물학적
- 물리적
- 인간공학적
- 사회/심리적
Employee Health Management Programs
SK bioscience supports various programs aimed at improving the health of employees. Its ‘Mind-Body Control Training’ is a corporate culture program that promotes healthy bodies and ambition, which is one of the core values of the SK Group. It offers employees various health management methods such as Miracle Morning, yoga and meditation. We also provide regular health check-ups for employees. Our health administration office is staffed by in-house nurses who conduct health assessments to identify any abnormalities, and we provide separate management for employees with medical conditions. The in-house health management office includes a health consultation room, a pharmacy that provides over-the-counter medications, a treatment room for minor injuries, and a recovery room, offering both health consultations and treatment convenience for employees.
We maintain a list of nearby emergency hospitals for immediate medical treatment in the event of work-related accidents. Additionally, we collaborate with specialized examination center hospitals to conduct general and specialized medical examinations at least once a year. Furthermore, we offer comprehensive health check-up services for employees and their family members (spouses). In addition, programs to support smoking cessation and weight control, aiming to help our employees protect themselves from health hazards are provided. We also provide annual flu and zoster vaccination to prevent infectious diseases among employees and their families.
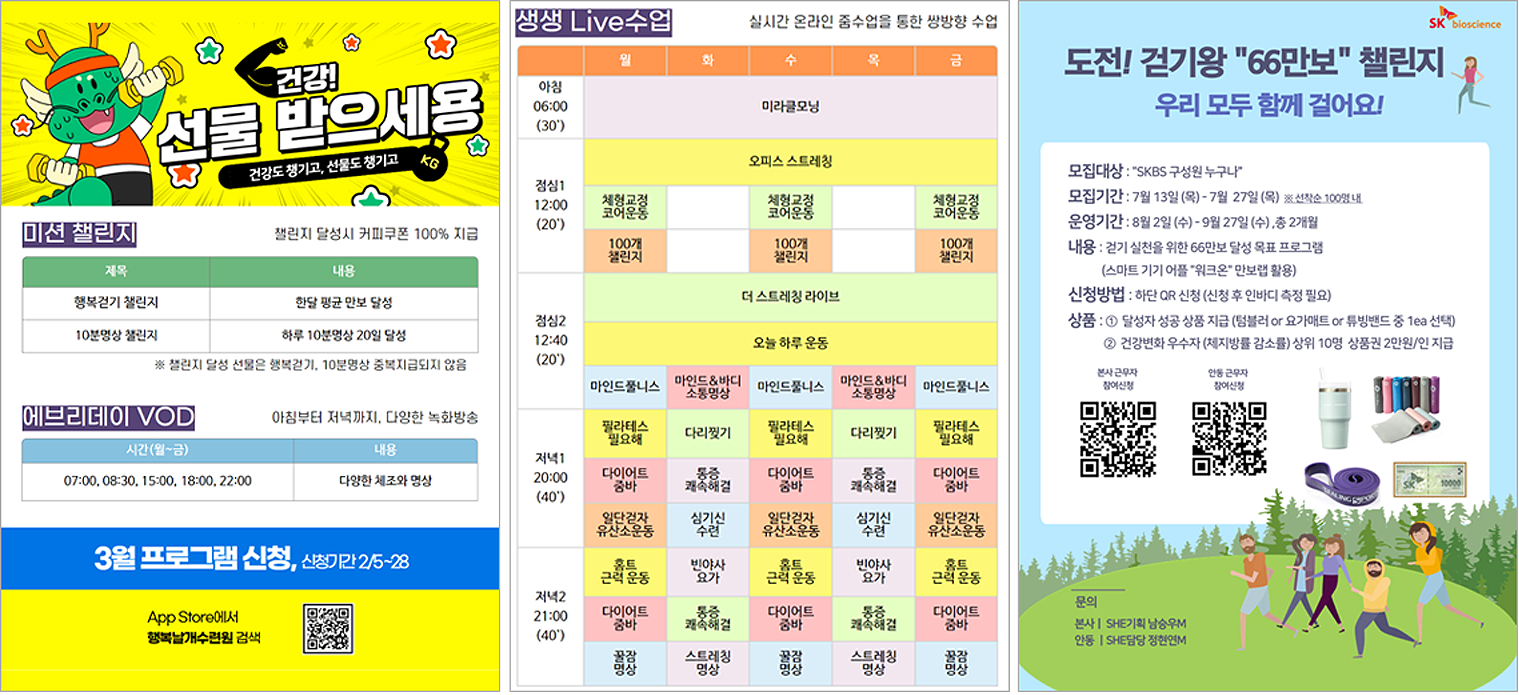

Mind-Body Control Training Program Poster and Timetable
Health Friendly Corporate Certificate
As a healthcare company, SK bioscience creates a health-friendly culture and environment to promote the health of employees and provides various supports for employees to manage their own health. As a result of these efforts, we were certified as a health-friendly company in 2024, and also received an award from the Ministry of Health and Welfare as an outstanding company.



Supplier Safety & Health Management
Safety & Health Cooperation Programs
SK bioscience extends safety and health management activities to business partners, serving as a reliable supporter for their safety and health management. We conduct safety and health trainings for all workers at our worksites, including workers from resident contractors, to communicate SHE compliance at work.
Examination of SHE Qualification
SK bioscience conducts SHE qualification examinations to strengthen the level of safety and health of suppliers within workplace and restricts the work of suppliers that are deemed unqualified as a result of the examination to encourage voluntary investment and improvement in safety and health.
From 2023, we have mandated the supplier qualification examinations, quarterly checking the safety and health levels of our suppliers and reflecting the results when contemplating additional contracts. We conduct a quantitative assessment based on the possession of systems for each of the seven items required by the assessment. In addition, we are establishing a plan to identify and manage potential risks that may occur during work by requiring suppliers to submit safety management plans in advance, including pre-work risk assessments and safety management plans.
Evaluation Criteria for Supplier SHE Qualification
- Safety & Health Management Policy
- Safety & Health Regulation and Procedure
- Presence of Safety & Health Management Team/Personnel
- Establishment & Implementation of Safety & Health Training Plans
- Development of Detailed Plans for On-site Safety & Health Management
- Hazardous and Risky Machines/Equipment Usage & Inspection Plan
- Occurrences of Accidents in the Past Three Years
Communication with Suppliers regarding Safety & Health
SK bioscience maintains ongoing communication with stakeholders, including suppliers, regarding all safety and health-related matters that arise in the workplace. The Resident Business Partner Committee listens to the grievances of workers from suppliers regarding safety and health, and takes measures to resolve them through discussions on emergency response procedures, accident reporting systems, and safety and health practices during work.
