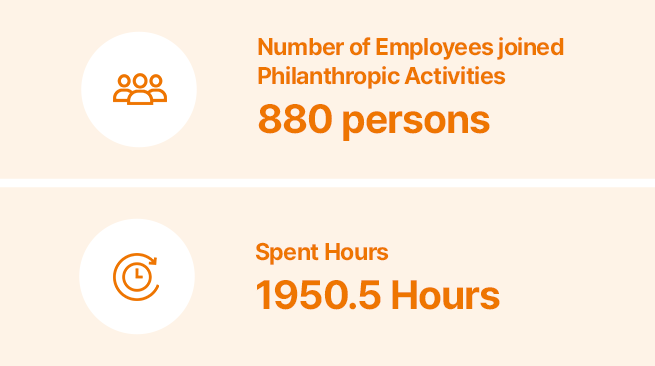
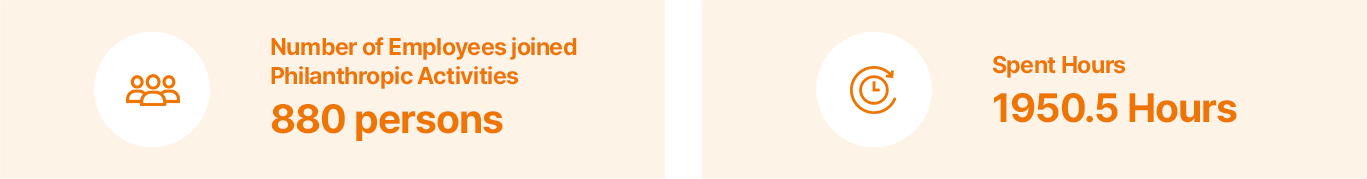Sustainable Supply Chain
Supply Chain Management System
SK bioscience shares ESG management values with business partners to build a sustainable and systematic supply chain in line with regulation changes at home and abroad, and builds strategic partnerships to strengthen competitiveness together.
Based on our supply chain ESG policy, we have developed various programs to promote mutual growth and enhance the capabilities of our business partners. We manage ESG risks across the entire value chain by diagnosing and supporting the implementation of improvements.

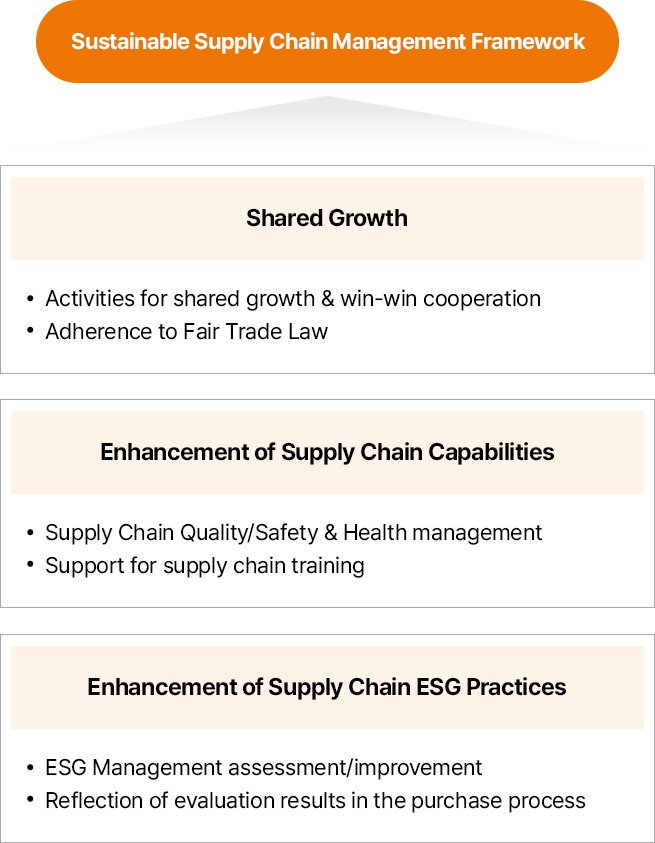
지속가능한 공급망 관리 체계
- 동반성장
- 상생협력 활동
- 공정거래 원칙 준수
- 공급망 역량 강화
- 협력사 품질/안전보건 관리
- 협력사 교육 지원
- 공급망 ESG 강화
- ESG경영 진단/개선
- 구매 과정 내 평가 결과 반영
Supply Chain Scope & Classification
As the Company that researches, develops, and produces biomedicine such as vaccines, SK bioscience has established strict standards to evaluate and register suppliers.
Main Suppliers
Companies with high strategic significance based on annual ordering costs or the nature of partnership in case of the key component suppliers, irreplaceable companies, and strategic procurement outsourcing suppliers
Other Suppliers
Companies that require a systematic management approach due to a significant business relationship with us
Supply Chain ESG Policy
SK bioscience requires all business partners to sign the ‘BP (Business Partners) Ethical Practice Pledge' prior to contract finalization to ensure the establishment of ethical and transparent business relationships.
Additionally, SK bioscience implemented a supply chain ESG policy in 2022. The Company has also established a 'Code of Conduct for Partners,' providing guidelines to partners for ESG management practices and encouraging compliance through a commitment pledge.
Supplier Code of Conduct
- Adoption of supplier code of conduct which is comprised of five sections (Human Rights and Labor, Safety & Health, Eco-friendliness, Business Ethics, Management System)
ESG Self-checklist
- Self-inspection of check-list items such as fair trade/anti-corruption, safety issues, and partner transaction status for all purchase contracts
Compliance (CP) Agreement
- Ensuring transparency in transactions and the practice of business ethics concerning business partners through
- Inclusion of the ‘BP ethical practice pledge’ in the initial contract
- Business legitimacy and contract status checks before and after the agreement
Code of Ethics for Purchasing
- Basic principles of fair and transparent transactions
- Compliance with laws and international conventions (The Universal Declaration of Human Rights, UN Global Compact, etc.)
- Reporting of violations and protection of the informant
Bid Evaluation Guide
- Partner selection principles, bidding process, detailed guidelines containing ESG elements
Social-responsibility-based Purchasing Principles
- Fulfillment of corporate social responsibility by pursuing shared growth in selecting trading partners and reviewing/supporting business partners’ non-financial status
Shared Growth with Business Partners
SK bioscience forges collaborative relationships with partners based on transparency and fairness, driving co-growth through various policies and support programs. To this end, the Company enforces strict adherence to legal regulations and guidelines that safeguard partner rights from the procurement bidding stage to contract finalization. SK bioscience complies diligently with all relevant laws, including the Monopoly Regulation and Fair Trade Act, the Act on Fair Transactions in Subcontracting, and other pertinent regulations. The Company also implements fair and transparent contract guidelines to advance co-growth within the biochemical industry.
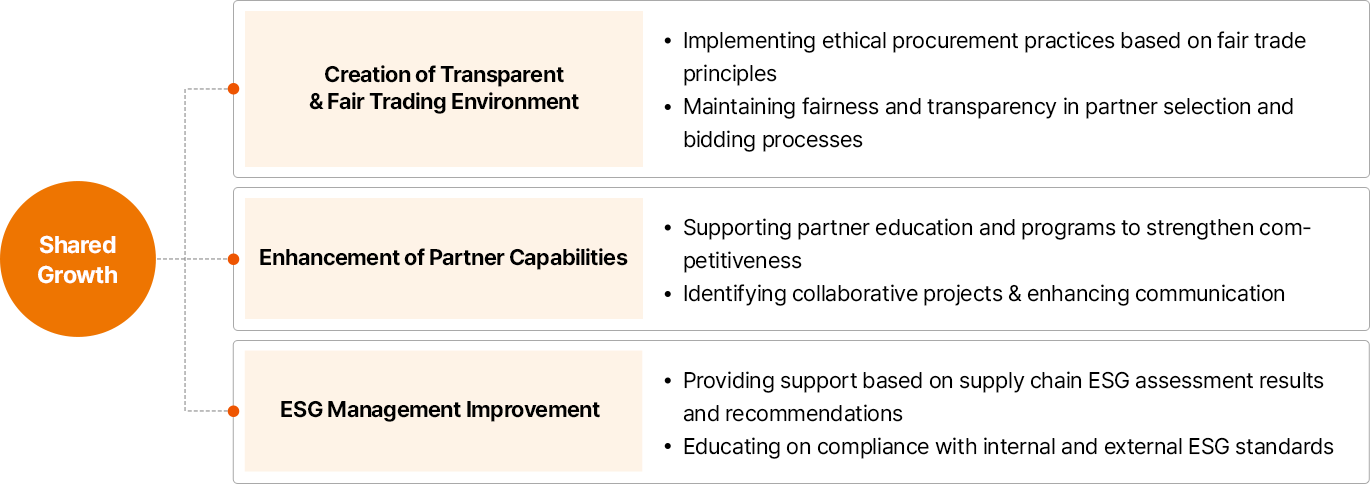

동반성장
- 투명/공정한 거래환경 조성
- 공정거래 원칙 기반 윤리적 구매 실천
- 협력사 선정 및 입찰 프로세스 공정 기회/ 투명성 유지
- 협력사 경쟁력 강화
- 협력사 교육/경쟁력 강화 프로그램 지원
- 협업 과제 발굴 및 소통 강화
- 협력사 ESG 경영 지원
- 공급망 ESG 진단 결과 기반 개선안 지원
- 대내외 ESG 기준 이행 위한 교육
Enhancement of Business Partner Capability
SK bioscience bolsters business partners' competitiveness through the SK Shared Growth Academy by offering education and training programs. In the future, we will operate an Open-Lab at our Songdo Global R&PD Center to strengthen our support system for supplier growth.
Additionally, SK bioscience extends its quality management and occupational health and safety management activities across the supply chain to strengthen partners' quality and safety competencies. Regular audits and evaluations are conducted to improve quality systems for primary, secondary, and tertiary partners. The Company also provides safety and health training for on-site partner employees to enhance safety capabilities.
To further strengthen partners' ESG management capabilities, SK bioscience began conducting supply chain ESG assessments in 2023. Based on the assessment results, improvement plans are developed and implementation support is provided for each partner. The Company plans to expand educational programs for partners to elevate ESG management and keep information updated.
Enhancement of Communication Channels
To listen to and actively communicate with suppliers’ grievances, SK bioscience operates a grievance channel in the procurement portal, along with various communication channels such as mail, phone, and video conferencing. We also resolve disputes related to the Code of Ethics through the SK Group Ethical Management Reporting Channel and strictly guarantee the confidentiality of the content of the report and the identity of the informant. In addition, we plan to establish fair trade practices and enhance transparency throughout the supply chain by operating the Voice of Suppliers for secondary and tertiary suppliers.
Supply Chain ESG Risk Management
Supply Chain ESG Risk Assessment Process
SK bioscience has established an online inspection system to identify and improve potential ESG risks within the supply chain based on Supplier Code of Conduct and global guidelines. In 2024, the Company selected assessment targets by comprehensively considering transaction amounts and frequencies over the past three years, the result of 2023 supply chain ESG risk assessment, and criticality of ESG in business. The entire process included self-assessment, third-party verification, on-site inspections, and consulting to develop improvement tasks.
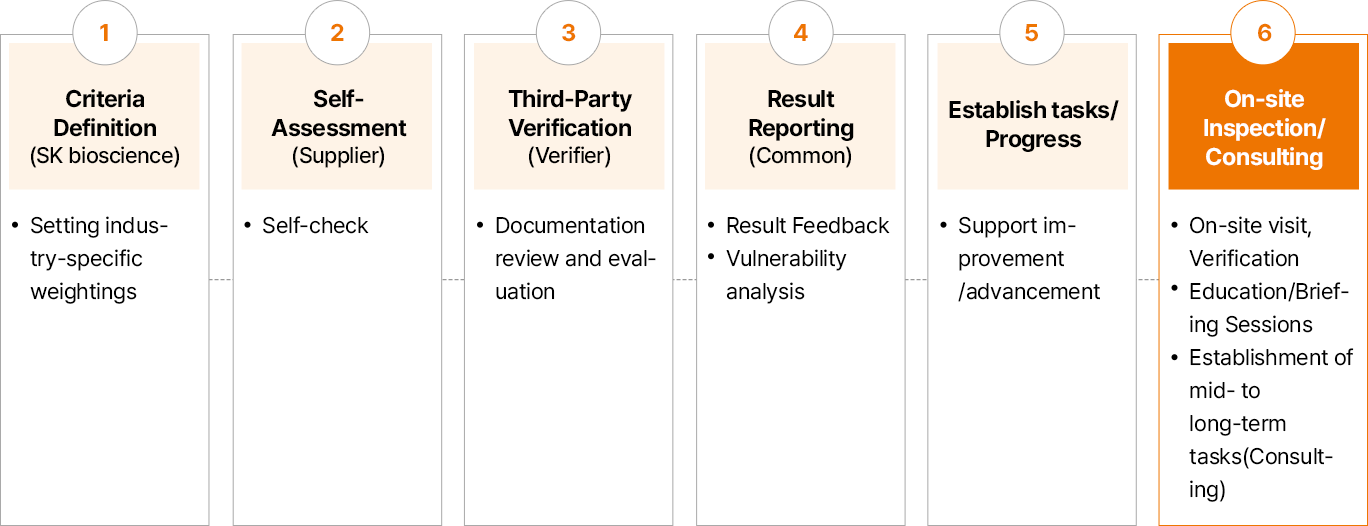
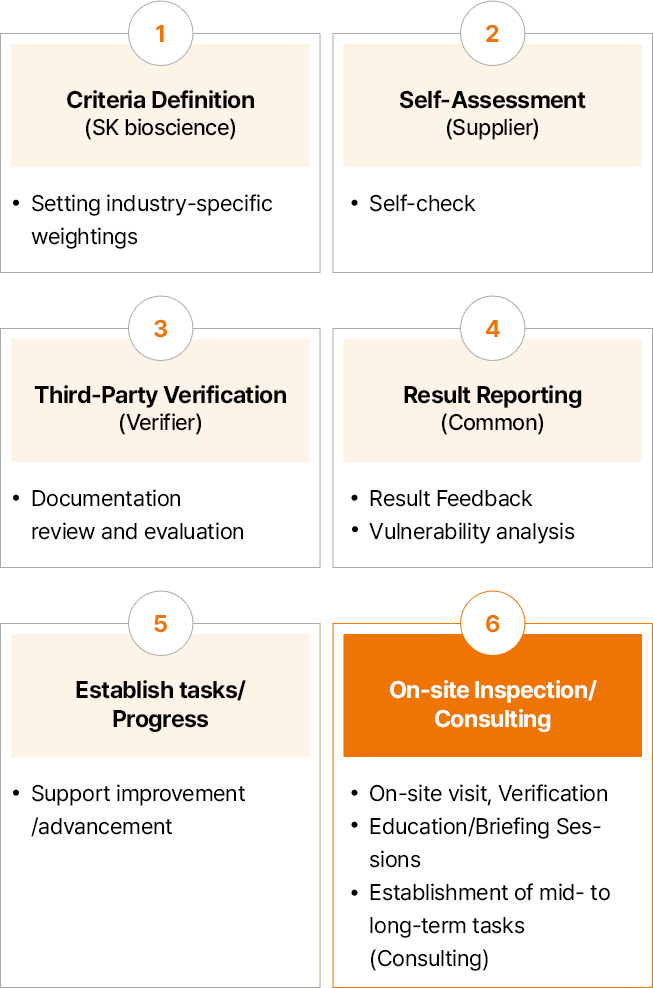
- 기준 정의 (원청사) : 산업군별 가중치 Setting
- 자가진단 (협력사) :Self-check
- 3자 검증 (검증사) :증빙 검토 및 평가
- 결과 Report (공통) :결과 Feedback, 취약점 분석
- 과제 수립/ Progress :개선/고도화 지원
- 현장실사/컨설팅 현장방문, 검증 교육/설명회 중장기 과제 수립(컨설팅)
In 2024, SK bioscience completed supply chain ESG risk self-assessments and third-party verifications for 44 partners. The assessment results did not identify any legal violations in major items; however, approximately 68% of participating companies were identified as high-risk, thus conducted a comprehensive improvement consulting to establish focused improvement tasks for early resolution of ESG risks and prioritize those tasks for each business partner.
In 2025, we plan to extend the supply chain ESG risk assessment process to advance our partners' risks management capabilities and build a solid sustainable supply chain system.
Assessment Indicators & Risk Evaluation Measures
The supply chain ESG management assessment indicators consist of 60 indicators on policies, practices, and performances in respective ESG areas and 6 indicators of legal violations by integrating domestic and international disclosure and assessment indicators. Given the impacts and ramifications of ESG issues varying by industries, the assessment weightings for respective ESG areas are applied differently.
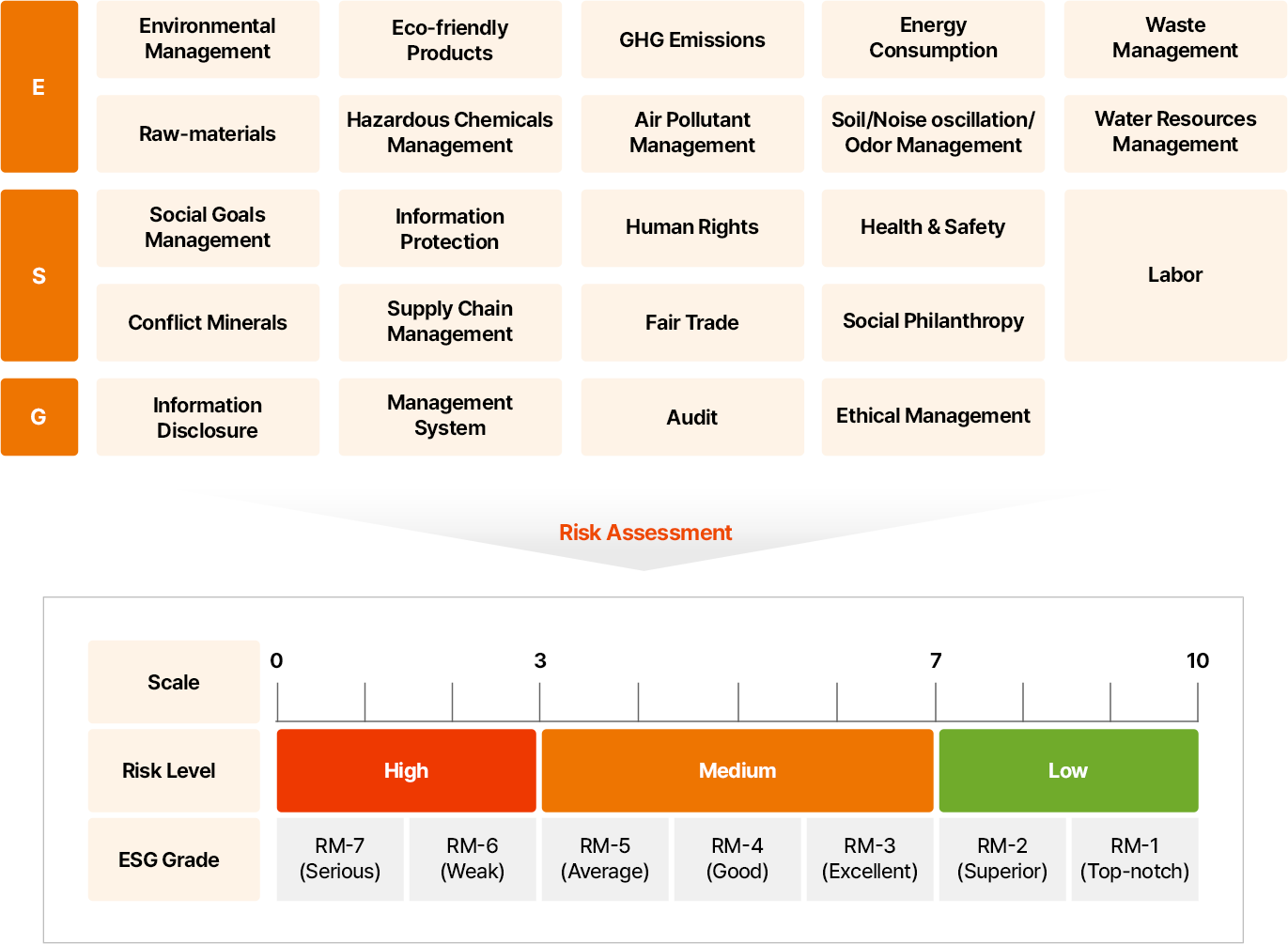
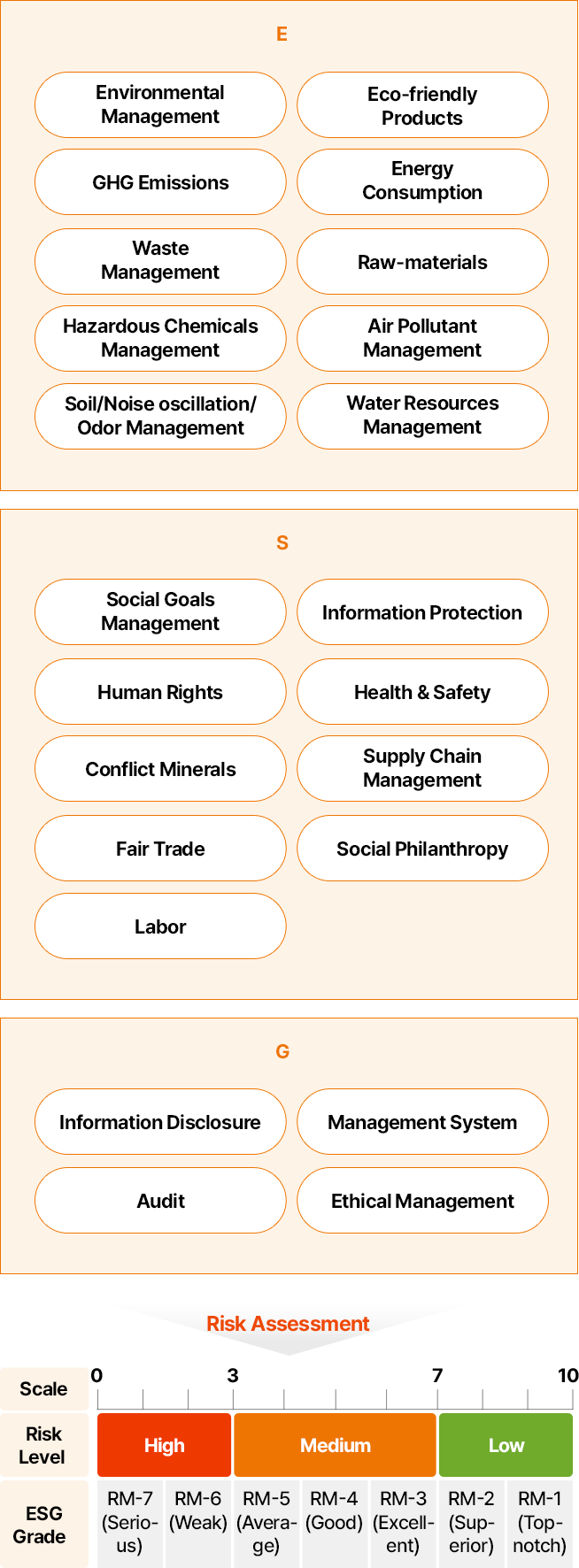
- E
- 환경경영 관리
- 친환경 제품
- 온실가스 배출
- 에너지 소비
- 폐기물 관리
- 원부자재
- 유해물질 관리
- 대기오염물질
- 토양/소음진동/악취
- 수자원보호
- S
- 사회 목표 관리
- 정보보호
- 인권
- 보건안전
- 분쟁광물
- 협력사 관리
- 공정거래
- 사회공헌
- 노동
- G
- 정보 공시
- 관리시스템
- 감사
- 윤리경영
리스크 평가
Support for Improving Supply Chain ESG Risk Management
In 2024, SK bioscience disseminated the results of its supply chain ESG risk assessment to participating entities and provided comprehensive improvement consulting for high-risk suppliers. The Company pinpointed key management targets and indicators, devised a risk mitigation plan to elevate all targeted suppliers out of the high-risk category, and systematically monitored the progress of these implementations.
Additionally, SK bioscience executes annual ESG management training for participating suppliers before conducting the supply chain ESG risk assessment. In 2024, the training encompassed general ESG management principles, the necessity of environmental management, disclosure practices, case studies, policies from other entities, and diagnostic guidelines to amplify the efficacy of supply chain ESG risk mitigation. The Company also assisted the consulting partners in setting environmental management objectives and tracking their performance since 2023. These improvement efforts resulted in 50% of high-risk partners engaged in consulting in 2023 improved their risk diagnosis to medium risk in the 2024 diagnosis.
In 2025, SK bioscience intends to enhance the current consulting and educational programs, focusing on diversifying and optimizing risk mitigation strategies. This includes systematically monitoring the implementation of improvement plans for key management suppliers, and broadening educational support. Especially, the Company will meticulously evaluate the ESG management training requirements of suppliers involved in the supply chain ESG risk assessment, continuing to offer proactive support through information dissemination, linking to external educational resources, and providing other essential assistance for effective ESG management within the biochemical industry.
IDT ESG Risk Management in the Supply Chain
The supply chain is a key focus for IDT Biologika (hereinafter IDT) for two main reasons: compliance with the German Supply Chain Sustainability Act (hereinafter the Act) and the emphasis on sustainability in its purchasing practices.
IDT considers social, ecological, and environmental factors as critical criteria when choosing suppliers and service providers. Suppliers must prove that they meet relevant standards, such as ISO 14001, ISO 45001, and ISO 50001 certifications. These requirements are outlined in the supplier qualification documents.
IDT conducts risk-based assessments to verify that their standards align with IDT's high expectations. The risk analysis, in accordance with Section 6 of the German Supply Chain Due Diligence Act, involves a comprehensive review of compliance with internationally recognized human rights and environmental standards. As part of the Act requirements, a full risk assessment was conducted in 2024, and this assessment will be repeated annually. The results are submitted to the Federal Office of Economics and Export Control in the form of a report.
To ensure that sustainability principles are deeply integrated into procurement practices, a mandatory annual training course on "Sustainability in Procurement" has been implemented for all procurement employees. This training helps ensure that purchasers are equipped to make responsible decisions and actively contribute to achieving IDT's sustainability goals.
Enhancing Access to Healthcare
Fostering Global Bio Ecosystem
Supporting Shared Growth of the Global Bio Industry
To contribute to global public health, SK bioscience supports the growth of stakeholders in the bio-industry to promote shared growth in each area. We will continue to strive to become a company that generates social value through investments in bio ventures, collaborative research and development to advance bio technologies, nurturing professionals, and supporting global initiatives and local communities.
Supporting the Growth of Bio-ventures
SK bioscience has invested in the KRW 150 billion Yuanta K-Bio Vaccine Fund to support the growth of bio ventures in the clinical stage and contribute to a creation of a bio ecosystem. Along with the fund investment, SK bioscience also directly invests in a number of domestic bio ventures to promote mutual growth.
Investment Review Process


- 신규 EV Item 발굴:유관 조직
- 투자심의위원회 실시
- ESG위원회 보고:전략기획실/이사회사무국
- 이사회 보고:전략기획실/이사회사무국
- Item 시행:유관 조직
투명한 의사결정을 위한 실행 프로세스 강화
Basic Research Support
SK bioscience collaborates closely with the International Vaccine Institute (IVI) in pursuit of our shared objective to promote 'global public health'.
We have pledged to donate KRW 3 billion to IVI for three years from 2022 to support vaccine R&D equipment and research. In this way, we aim to make further advancements to the vaccine R&D ecosystem by supporting organizations involved in all stages of the value chain, from development to distribution of vaccines.
SK bioscience has also established an industry-academia collaboration system for infectious disease response at Korea University Medical Center in 2022. Also we have allocated KRW 5 billion for three years (year 2022 to 2025) to establish an infectious disease surveillance system and conduct joint vaccine research.
Hosting Park Mahn-Hoon Award Ceremony
In honor of the dedication to vaccinee R&D, SK bioscience, together with the International Vaccine Institute, established the Park Mahn-hoon Award. This award aims to recognize individuals and organizations for their outstanding contributions to global health and enhance the global vaccine R&D capabilities. In April 2024, the third Park Mahn-hoon Awards were presented to Professor Jan Holmgren at the University of Gothenburg, Sweden, who have developed the world's first oral cholera vaccine, and Professor Barney Graham at Morehouse School of Medicine, USA, and Professor Jason McLellan at the University of Texas, USA, who have contributed to the rapid development of COVID-19 vaccines and antibody therapies through their research in antigen design, reagent development, vaccine delivery and manufacturing technologies.
| RIGHT Fund sponsorship | International Vaccine Institute (IVI) sponsorship | Research support |
|---|---|---|
|
Empowerment through the IVI network and expertise
|
Support for advances in basic research
|
Fostering Global Bio-talent
Fostering Talent through Scholarship Support
SK bioscience supports research in the healthcare field through collaborations with various global organizations and sponsorship with the goal of ‘promoting public health worldwide’. In addition, we foster global bio-talent by providing scholarships and sponsoring educational programs for exceptional individuals in the field of bio/vaccines.
-
Park Mahn-Hoon Scholarship
- Department of Life Sciences at Seoul National University, Boseong High School
: Granting about 10 students with KRW 5 million every year
- Department of Life Sciences at Seoul National University, Boseong High School
-
Other Scholarships
- Gyeongkuk National University
: Granting 8 students with KRW 2 million every year - Supporting the Department of Vaccine Biotechnology of the Gyeongkuk National University
: Providing mentoring, education and collaborative projects, field training, curriculum collaboration, and internship experience
- Gyeongkuk National University

Gyeongkuk National University’s Industry-Academia Cooperation Ceremony
Global Training Hub for Biomanufacturing (GTH-B)
As a partner organization of the World Health Organization (WHO), SK bioscience is dedicated to nurturing the next generation of bio-talent through supporting educational operation. Since Korea was selected as a WHO's Global Training Hub, we have been participating in regular training courses since 2022 and strengthening a mutual cooperation to train vaccine and bio professionals.
We have also sponsored the International Vaccinology Course (IVC) organized by the International Vaccine Institute (IVI) for the past four years, providing specialized training to 192 global trainees from 35 countries as of 2024. In addition, our members are also trained on-site and actively participate in building a global network of vaccine experts.
Through these activities, SK bioscience strengthens its global network and fulfills its role as a ‘global training hub for biomanufacturing’, ultimately contributing to sustainable development of vaccine and bio industry.
Market Expansion & Support Strategy
Board/Committee Oversight for Access to Healthcare Issues
SK bioscience regularly reviews and oversees issues related to the access to healthcare at the Board of Directors and ESG Committee level to promote global public health and reduce the imbalance in vaccine supply. In 2024, we discussed in depth the agendas for various projects aimed at creating a global bio ecosystem.
| Category | Date | Agenda |
|---|---|---|
| ESG Committee | February 6, 2024 | Report on operating status of Global Bio Ecosystem |
| ESG Committee | July 12, 2024 | Report on operating status of Global Bio Ecosystem |
Enhancing Access to Healthcare through Global Vaccine Supply
SK bioscience is contributing to the improvement of human health by building a value chain that encompasses the entire process from basic research to production, licensing, and commercialization of vaccines. In collaboration with global organizations, we have developed our own vaccine portfolio, SKYVAX, targeting infectious diseases that pose a significant burden on public health, including influenza, shingles, chickenpox, and typhoid.
SKYVAX products are essential vaccines for the prevention of infectious diseases that have a significant impact on public health, including influenza, which affects up to 1 billion people annually; chickenpox, which affects 140 million people; and typhoid, which has an incidence of 11 to 21 million cases. In particular, SKYTyphoid (typhoid vaccine), developed in collaboration with the International Vaccine Institute (IVI) with support from the Gates Foundation, is a prime example of a successful global collaboration that has been commercialized.
Global Expansion with Multilateral Procurement
SK bioscience is participating in international procurement biddings for SKYCellflu (influenza vaccine) and SKYVaricella (chickenpox vaccine), which have received WHO Prequalification (PQ), and continues to expand direct access to low- and middle-income countries (LMICs).
| Category | Content |
|---|---|
| PAHO1) |
|
| UNICEF |
|
| Asia Region |
|
- 1)PAHO : Pan American Health Organization (the American Regional Office (AMRO) of WHO)
Plan for Vaccine Supply Expansion
SK bioscience will contribute to improving human health by expanding global vaccine access and continuing international cooperation to prevent infectious diseases.
| Year | Countries (No.) | |
|---|---|---|
| SKYCellflu | SKYZoster & SKYVaricella | |
| 2024 | 9 | 16 |
| 2025 | 17 | 19 |
| 2026 | 28 | 24 |
| 2027 | 32 | 31 |
Global Partnership Collaboration
SK bioscience collaborates with global health partners such as the Gates Foundation, the International Vaccine Institute (IVI), GAVI4), CEPI5), and others to promote public health and improve access to health. These partnerships are focused on expanding vaccine supply in low-income countries and health-fragile regions and strengthening the global vaccine supply system.
In collaboration with the Gates Foundation, we are creating an innovative vaccine development and supply strategy that enables more effective and rapid production by sharing resources and technologies during the research and development of vaccines. This contributes to expanding vaccine supply to low-income countries and strengthen health systems.
Our collaborative research projects with the International Vaccine Institute (IVI) are playing a critical role in improving the effectiveness of vaccines and developing new vaccine candidates.
Our partnership with GAVI is focused on expanding the access to vaccines in low-income countries and driving efficiencies within the global supply chain, while our work with CEPI is focused on building a platform for rapid response in the event of an epidemic and improving access to healthcare.
Those global partnerships are a key foundation for SK bioscience's contribution to building a sustainable public health environment, and we will continue to work with various stakeholders to ensure that the world's population has access to safe and effective vaccines.
- 4)GAVI : Global Alliance for Vaccines and Immunization
- 5)CEPI : Coalition for Epidemic Preparedness Innovations
Glocalization Strategy
The global vaccine supply imbalance is a serious problem. To date, approximately 67% of the population in low- and middle-income countries (LMICs) have not received a single dose of COVID-19 vaccine, and according to the WHO's World Vaccine Market Report, the human papillomavirus (HPV) vaccine, a vaccine to prevent cervical cancer, has an uptake rate of 83% in developed countries, compared to less than half in LMICs at 41%.
To address these issues, SK bioscience is developing glocalization projects around the world. Glocalization is an innovative model that goes beyond the concept of establishing local factories to produce products, and promotes self-sufficiency of each country's vaccines to ensure timely supply of the vaccines they need. We are continuing discussions with governments and local companies in various countries, and plan to expand the project gradually.
First, we are promoting the localization of vaccine development and production with the Thai government based on a Public-Private Partnership. We are working closely with the Thai government's state-owned pharmaceutical company and relevant government agencies with the goal of localizing vaccines in Thailand and strengthening health security in the ASEAN region.
Based on this collaboration, we are discussing long-term cooperation utilizing SKYVAX and other vaccine-based technologies. This will enable SK bioscience to secure a stable supply chain in Thailand and the ASEAN region, and contribute to improving vaccine self-sufficiency and pandemic preparedness in the region.
Supporting Capacity-Building in Developing Countries
SK bioscience participates in the Regional Vaccine Manufacturing Collaborative (RVMC) to address global vaccine inequalities and strengthens access to vaccines. The RVMC is a global council launched at the World Economic Forum (WEF) Annual Meeting in May 2022 to address the inequality in vaccine supply between high-income countries and low- and middle-income countries during the COVID-19 pandemic. SK bioscience is working with global public health organizations such as WHO and GAVI to establish regional vaccine hubs and create a data-driven, sustainable global supply chain and collaborative ecosystem. In doing so, we strive to strengthen global partnerships and contribute to improving the global vaccine supply chain.
We also actively supports our partners in developing countries to strengthen their pharmacovigilance (PV) capabilities. Beyond PV audits on our international partners, we also help our partners in low- and middle-income countries (LMICs) understand advanced pharmacovigilance systems, with experts from both our audit and PV teams visiting them to provide practical advices.
In addition, starting in 2024, we are offering PV training for our international partners on our products. The training is more than just information transfer. It is focused on enabling our partners in LMICs to learn advanced PV business systems and know-how and apply them in their works.
Based on our experience and know-how in vaccine R&D and production, SK bioscience is engaged in business discussions with governments and local companies in various countries to provide technologies to other countries with poor vaccine infrastructure and to build local production facilities so that they can develop their own production capabilities. We plan to support our international partners in strengthening their infrastructures and production capabilities, including meeting international drug manufacturing standards, by helping them build production infrastructure, sharing know-how, dispatching our technical personnels for technology transfer, and training local personnels.
Affordability
Increasing Affordability across Markets
SK bioscience supplies its vaccine products, including SKYVAX, to both public and private markets in Korea and abroad. In the public market, we supply vaccines to various countries, including Korea and low- and middle-income countries (LMICs), through participation in the National Immunization Program (NIP) and multilateral procurement projects.
When pricing vaccines, we consider a variety of factors, including market conditions, cost structures, policies and regulations, and the public good nature of vaccines, while ensuring that market-specific price accessibility is fully considered. The key factors to consider are as follows.
- Increasing vaccine access: In LMICs, we're adjusting prices or offering special prices to help more people get vaccinated.
- Partnership & collaboration: We work with international organizations such as GAVI and WHO to increase global vaccine access by aligning vaccine pricing and distribution strategies.
We participate in a variety of multilateral procurement projects to improve access to healthcare in low-income countries, contributing to the local supply of essential medicines at affordable prices. For SKYCellflu and SKYVaricella which we supply through PAHO, we use PAHO's procurement prices, which are updated twice a year and shared with us, as a guide for pricing. Similarly, for SKYTyphoid, which we are preparing to supply through UNICEF, we look at the supplier prices published by UNICEF and set our prices accordingly.
In addition, private markets at home and abroad are setting the lowest public bids for each product and setting the right price, taking into account the needs and specificities of each market and the social value of universal vaccine access.